Question
Question: Find if Polar or Non Polar?...
Find if Polar or Non Polar?
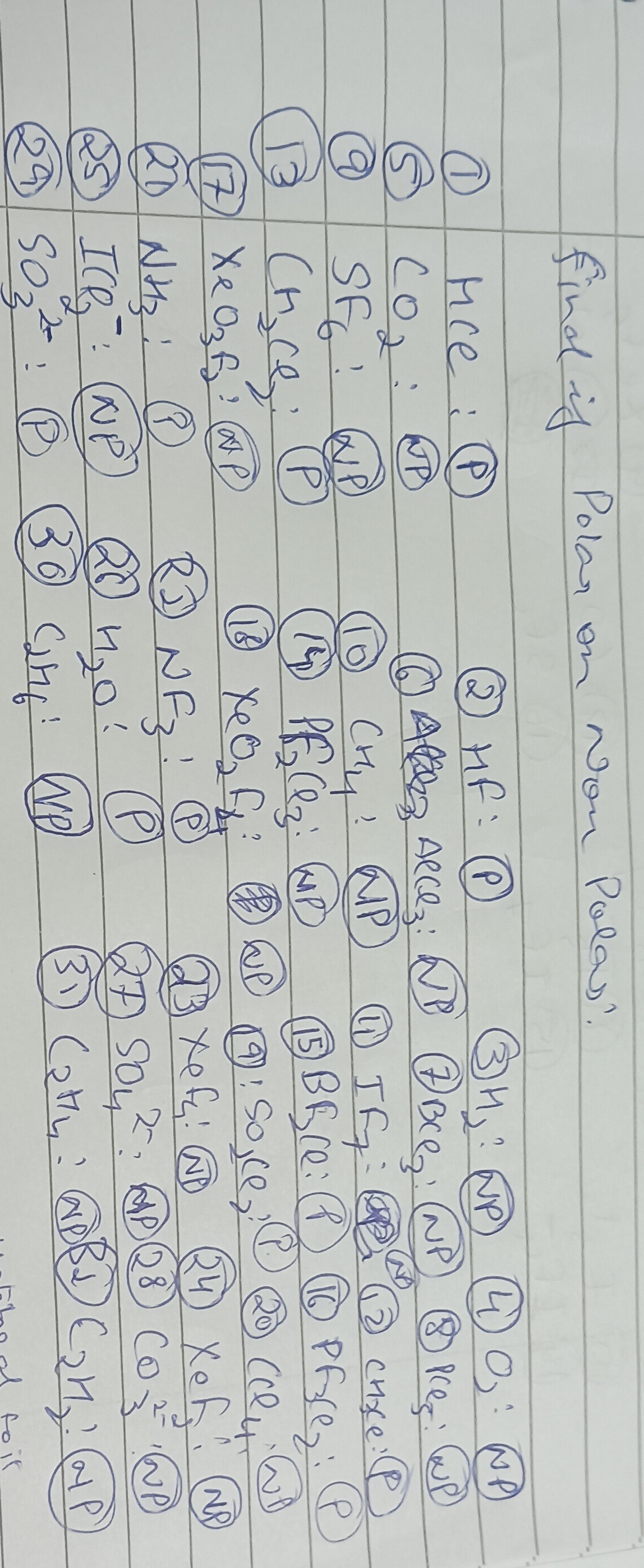
Answer
No specific option to choose as it is a list of molecules and their properties.
Explanation
Solution
To determine if a molecule is polar or nonpolar, we need to consider two main factors:
- Bond polarity: Whether the individual bonds within the molecule are polar due to a difference in electronegativity between the bonded atoms.
- Molecular geometry: Whether the arrangement of these polar bonds and any lone pairs on the central atom results in a net dipole moment for the entire molecule. Symmetrical molecules often have their bond dipoles cancel out, leading to a nonpolar molecule, even if individual bonds are polar. Asymmetrical molecules, or those with lone pairs on the central atom, are typically polar.
Let's analyze each molecule:
① HCl:
- Bond: H-Cl bond is polar (Cl is more electronegative).
- Geometry: Linear.
- Net dipole: Yes, the dipole points towards Cl.
- Polar (P)
② HF:
- Bond: H-F bond is highly polar (F is much more electronegative).
- Geometry: Linear.
- Net dipole: Yes, the dipole points towards F.
- Polar (P)
③ H₂:
- Bond: H-H bond is nonpolar (no electronegativity difference).
- Geometry: Linear.
- Net dipole: Zero.
- Nonpolar (NP)
④ O₃ (Ozone):
- Structure: Bent (V-shaped) with resonance. The central oxygen atom has one lone pair.
- Net dipole: Due to its bent geometry and the presence of formal charges/resonance, it has a net dipole moment.
- Polar (P)
⑤ CO₂:
- Structure: Linear (C=O bonds).
- Net dipole: C=O bonds are polar, but their dipoles are equal and opposite, canceling each other out in the linear geometry.
- Nonpolar (NP)
⑥ AlCl₃:
- Structure: Trigonal planar. No lone pairs on central Al.
- Net dipole: Al-Cl bonds are polar, but the symmetrical trigonal planar arrangement causes the dipoles to cancel.
- Nonpolar (NP)
⑦ BrCl₃:
- Structure: T-shaped (central Br has two lone pairs).
- Net dipole: Br-Cl bonds are polar. The T-shaped geometry is asymmetrical, and the lone pairs contribute to a net dipole moment.
- Polar (P)
⑧ PCl₅:
- Structure: Trigonal bipyramidal. No lone pairs on central P.
- Net dipole: P-Cl bonds are polar, but the symmetrical trigonal bipyramidal arrangement causes the dipoles to cancel.
- Nonpolar (NP)
⑨ SF₆:
- Structure: Octahedral. No lone pairs on central S.
- Net dipole: S-F bonds are polar, but the highly symmetrical octahedral arrangement causes the dipoles to cancel.
- Nonpolar (NP)
⑩ CH₄:
- Structure: Tetrahedral. No lone pairs on central C.
- Net dipole: C-H bonds are slightly polar, but the symmetrical tetrahedral arrangement causes the dipoles to cancel.
- Nonpolar (NP)
⑪ IF₇:
- Structure: Pentagonal bipyramidal. No lone pairs on central I.
- Net dipole: I-F bonds are polar, but the highly symmetrical pentagonal bipyramidal arrangement causes the dipoles to cancel.
- Nonpolar (NP)
⑫ CH₂Cl₂:
- Structure: Tetrahedral.
- Net dipole: C-H and C-Cl bonds are polar. The molecule is asymmetrical due to different types of atoms (H and Cl) attached to the central carbon, leading to a net dipole moment.
- Polar (P)
⑬ CH₂Cl₂: (Duplicate of ⑫)
- Polar (P)
⑭ PFCl₃:
- Structure: Tetrahedral (central P, 1 F, 3 Cl).
- Net dipole: P-F and P-Cl bonds are polar and have different magnitudes. The molecule is asymmetrical, so the bond dipoles do not cancel.
- Polar (P)
⑮ BHCl₂:
- Structure: Trigonal planar (central B, 1 H, 2 Cl).
- Net dipole: B-H and B-Cl bonds are polar. The molecule is asymmetrical due to different atoms, leading to a net dipole moment.
- Polar (P)
⑯ PBrCl₂:
- Structure: Trigonal pyramidal (central P has one lone pair).
- Net dipole: P-Br and P-Cl bonds are polar. The lone pair and the asymmetrical arrangement of different atoms lead to a net dipole moment.
- Polar (P)
⑰ XeO₃F₂:
- Structure: Trigonal bipyramidal (central Xe, 3 O, 2 F). No lone pairs on central Xe.
- Net dipole: Xe-O and Xe-F bonds are polar. Even with F in axial and O in equatorial positions, the different bond types and their arrangement make the molecule asymmetrical, resulting in a net dipole moment.
- Polar (P)
⑱ XeO₂F₂:
- Structure: See-saw (central Xe has one lone pair).
- Net dipole: Xe-O and Xe-F bonds are polar. The see-saw geometry is asymmetrical, and the lone pair contributes to a net dipole moment.
- Polar (P)
⑲ SO₂Cl₂:
- Structure: Tetrahedral (central S, 2 O, 2 Cl).
- Net dipole: S=O and S-Cl bonds are polar. The molecule is asymmetrical due to different atoms, leading to a net dipole moment.
- Polar (P)
⑳ NH₃:
- Structure: Trigonal pyramidal (central N has one lone pair).
- Net dipole: N-H bonds are polar. The lone pair on N and the trigonal pyramidal geometry result in a net dipole moment.
- Polar (P)
⑳ ClO₂⁻: (Assuming this is a separate entry)
- Structure: Bent (V-shaped) (central Cl has two lone pairs).
- Net dipole: Cl-O bonds are polar. The lone pairs and bent geometry lead to a net dipole moment.
- Polar (P)
㉒ NF₃:
- Structure: Trigonal pyramidal (central N has one lone pair).
- Net dipole: N-F bonds are polar. The lone pair on N and the trigonal pyramidal geometry result in a net dipole moment.
- Polar (P)
㉓ XeF₄:
- Structure: Square planar (central Xe has two lone pairs in axial positions).
- Net dipole: Xe-F bonds are polar, but the symmetrical square planar arrangement and cancellation of lone pair dipoles result in no net dipole moment.
- Nonpolar (NP)
㉔ XeF₂:
- Structure: Linear (central Xe has three lone pairs in equatorial positions).
- Net dipole: Xe-F bonds are polar, but the symmetrical linear arrangement and cancellation of lone pair dipoles result in no net dipole moment.
- Nonpolar (NP)
㉕ ICl₂⁻:
- Structure: Linear (central I has three lone pairs in equatorial positions).
- Net dipole: I-Cl bonds are polar, but the symmetrical linear arrangement and cancellation of lone pair dipoles result in no net dipole moment.
- Nonpolar (NP)
㉖ H₂O:
- Structure: Bent (V-shaped) (central O has two lone pairs).
- Net dipole: O-H bonds are polar. The lone pairs and bent geometry lead to a net dipole moment.
- Polar (P)
㉗ SO₄²⁻:
- Structure: Tetrahedral (all S-O bonds equivalent by resonance).
- Net dipole: S-O bonds are polar, but the symmetrical tetrahedral arrangement causes the dipoles to cancel.
- Nonpolar (NP)
㉘ CO₃²⁻:
- Structure: Trigonal planar (all C-O bonds equivalent by resonance).
- Net dipole: C-O bonds are polar, but the symmetrical trigonal planar arrangement causes the dipoles to cancel.
- Nonpolar (NP)
㉙ SO₃²⁻:
- Structure: Trigonal pyramidal (central S has one lone pair).
- Net dipole: S-O bonds are polar. The lone pair on S and the trigonal pyramidal geometry result in a net dipole moment.
- Polar (P)
㉚ CH₄: (Duplicate of ⑩)
- Nonpolar (NP)
㉛ C₂H₄ (Ethene):
- Structure: Planar.
- Net dipole: C-H bonds are slightly polar, but the overall symmetrical planar structure causes the dipoles to cancel.
- Nonpolar (NP)
The question asks to "Find if Polar or Non Polar?". It implies providing the correct classification. The provided list in the image has several incorrect classifications.
