Question
Question: Find distance of centre of mass of \({{H}_{2}}0\) molecule....
Find distance of centre of mass of H20 molecule.
Solution
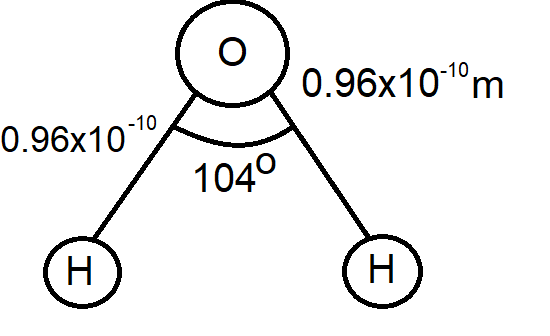
In order to solve this question, you must first know the structure of the structureH20 molecule. The H20 molecule is formed by the two hydrogen atoms and an oxygen atom. The distance between the hydrogen atom and oxygen atom is 0.96×10−10m. Forming an angle of 104∘.
The mass of one hydrogen atom is 1 unit and the mass of an oxygen atom is 16 units. Using these data, one can easily calculate the centre of mass by simply applying the same in the formula for the centre of mass.
Complete answer:
Before we start solving the question, let us take a look at the molecular structure of H20 molecule.
Now,
We can see that it is in form of a triangle
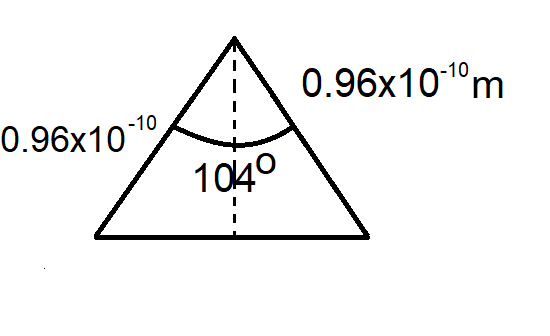
Now, as we know that
Mass of a hydrogen atom = 1 unit
And, the mass of one oxygen atom will be 16 unit
So, now
Let the distance between the hydrogen atom and oxygen atom be d
Then
d=0.96×10−10m
So,
By the formula,
XCM=m1+m2+m3m1+m2x2+m3x3
Now,
Using the values we have in the above formula, we have
⇒XCM=1+1+16−dsin52∘+dsin52∘+16×0
⇒XCM=0
Now,
⇒YCM=m1+m2+m3m1+m2y2+m3y3
⇒YCM=1+1+160+0+16×0.96×10−10cos52∘
⇒YCM=98×0.96×10−10cos52∘
So, we will have the coordinates of the centre of mass as (0, 98×0.96×10−10cos52∘)
Note:
In this question we have the answer in form of coordinates, it means the centre of mass is at a distance of 98×0.96×10−10cos52∘m away from the centre of the line joining the two hydrogen atom.
One interesting fact about The H20 molecule is that is forms hydrogen bond, which is a special type of bond only formed when a hydrogen atom is covalently bonded to a very electronegative atom such as a N, O, or F atom and another very electronegative atom.
