Question
Question: Find ‘A’ and ‘B’ in the given reaction sequence. \( C{H_3} - C \equiv CH\xrightarrow[{{\text{liq}...
Find ‘A’ and ‘B’ in the given reaction sequence.
CH3−C≡CHNaliq. NH3(A)CH3Cl(B)
Solution
Hint : When an alkyne reacts with sodium in liquid ammonia, it reduces to give a trans alkene. The reduction of alkynes with sodium in liquid ammonia is complementary i.e., opposite to the catalytic hydrogenation of alkynes in which cis alkene is obtained as a product. The intermediate involved in this reduction reaction is radical anion.
Complete Step By Step Answer:
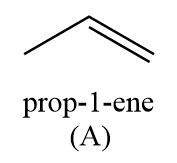
For the given reaction sequence, the structure of compound A in the given reaction will be as follows:
CH3−C≡CH2Naliq. NH3CH3−CH=CH2+2NaNH2
The mechanism for the reaction is given as follows:
Step-1: Sodium metal transfers an electron to the alkyne and formation of a radical-anion takes place. The reaction proceeds as follows:
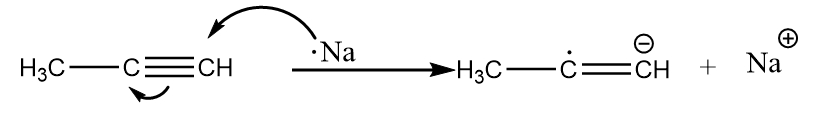
Step-2: The radical anion formed in the previous step attacks the ammonia molecule and removes the proton to undergo acid-base reaction. The reaction proceeds as follows:
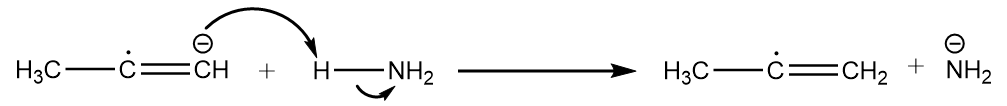
Step-3: Now, the second atom of sodium shares its electrons to the radical and formation of carbanion takes place. The reaction proceeds as follows:
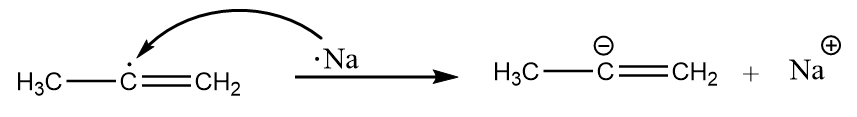
Step-4: The carbanion removes the proton from ammonia to give respective alkene. The reaction takes place as follows:
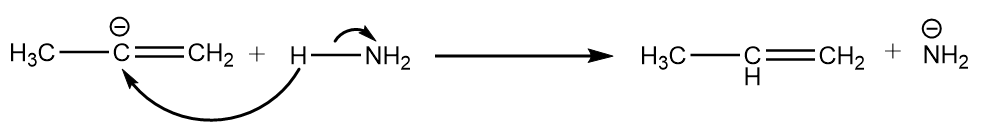
Hence, the compound ‘A’ formed is prop-1-ene.
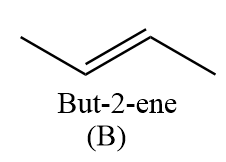
On further reaction of A with chloromethane, the structure of B formed will be as follows:
CH3−CH=CH2CH3ClCH3−CH=CH−CH3
The mechanism for the reaction is as follows:
Step-1: NaNH2 acts as a base and extracts acidic hydrogen from prop-1-ene and forms an intermediate along with the removal of ammonia. The reaction takes place as follows:
CH3−CH=CH2+NaNH2→CH3−CH=CHNa+NH3
Step-2: The intermediate formed in the previous step reacts with chloromethane and formation of but-2-ene takes place along with the removal of sodium chloride. The reaction takes place as follows:
CH3−CH=CHNa+CH3Cl→CH3−CH=CH−CH3+NaCl
Hence, the compound ‘B’ formed is but-2-ene.
Thus, we can conclude that in the given reaction sequence, the structure of ‘A’ and ‘B’ are as follows:
Note :
Remember that ordinary alkene does not react with solvated electrons or does not form a stable radical anion, so the reaction stops at the trans alkene stage i.e., no further reduction takes place after formation of the respective alkene.
