Question
Question: Fill in the blanks (B and C) with appropriate structures of reaction products in the following trans...
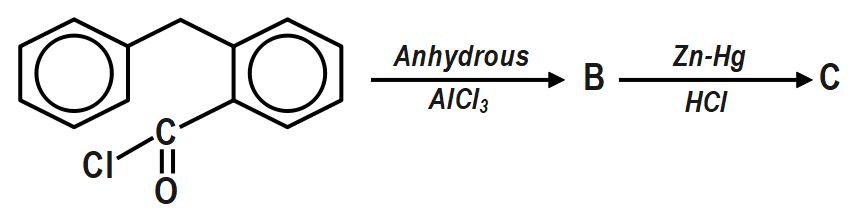
Fill in the blanks (B and C) with appropriate structures of reaction products in the following transformation:
Substitution pattern in Mon substituted Benzene Systems.
Solution
We know that there are two types of groups which are found to be attached with the benzene ring. It may be an Electron Withdrawing group or Electron Donating group because both shows have a different effect on the upcoming group or second substituent.
Complete answer:
Because there are two types of the substituent which attach to the benzene ring and show their affection, the position on which they are attached also plays a key role because it influences the ring. There are three-position mentioned in the aromatic ring i.e. ortho, para and Meta position. Ortho position is present adjacent to the functional group whereas Meta position is the position which is adjacent to the ortho position and para position is adjacent to the Meta position
The electron-withdrawing group has only one major group that is Meta position and causes the second substituent to attach to the Meta position. Whereas the electron-donating groups are those species which have a lone pair of an electron to donate to the neighboring atoms. For example; the alkyl group. The electron-donating group has two major groups that are ortho and para position and causes the second substituent to attach on either ortho or para position. So, we can say that the nature of the second substituent depends on the nature of the non-substituent group.
Note:
Remember that the mono-substituent benzene ring is formed by the process of Electrophilic Aromatic Substitution. The addition of the second substituent is also dependent on the resonance (delocalization of pi bond) and inductive (an electronic effect caused by the polarization of sigma bond) effects.
