Question
Question: ```text $\xrightarrow{H_2SO_4}$ + ```...
$\xrightarrow{H_2SO_4}$ +
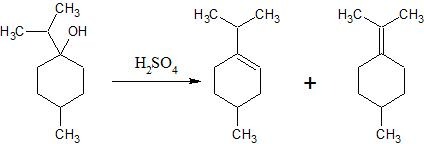
The reaction produces a mixture of two alkenes. The major product is the alkene with the isopropylidene group (C(CH₃)₂ attached to the ring via the double bond), and the minor product is the alkene with the isopropyl group (CH(CH₃)₂ attached to the ring via the double bond).
Solution
When the cyclic secondary alcohol is treated with H₂SO₄, it undergoes acid‐catalyzed dehydration via an E1 mechanism. The protonation of –OH forms water as a good leaving group, generating a carbocation intermediate. A proton is then removed from one of the two adjacent carbons leading to the formation of a double bond. Thus, two regioisomeric alkenes are formed. By Zaitsev’s rule, the more substituted alkene (where the double bond is conjugated with the isopropylidene group, i.e. C(CH₃)₂) is typically major, while the other alkene (with the isopropyl group, CH(CH₃)₂) is the minor product.
