Question
Question: $\xrightarrow{Conc. H_2SO_4 \\ \triangle}$ ...
Conc.H2SO4△
Answer
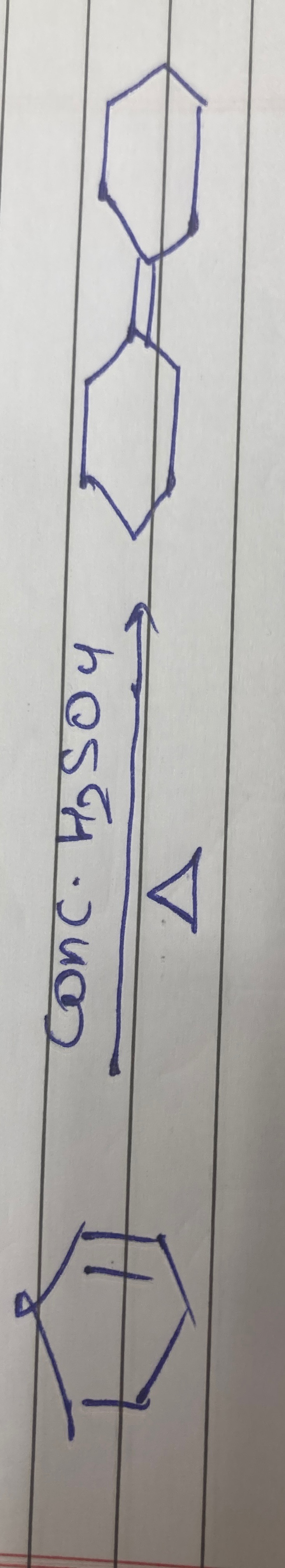
bicyclo[2.2.1]hept-2-ene
Explanation
Solution
The reaction is acid-catalyzed dehydration of exo-bicyclo[2.2.1]heptan-2-ol. Protonation of the alcohol is followed by loss of water to form a secondary carbocation at C2. Elimination of a proton from C3 leads to the formation of bicyclo[2.2.1]hept-2-ene (norbornene), which is a stable alkene. Although rearrangement to a bridgehead carbocation is possible, elimination from the initial secondary carbocation to form the stable internal alkene is the major reaction pathway.
