Question
Question: $\xrightarrow{CH_3-C-Cl}$ (A) (major) ...
CH3−C−Cl (A) (major)
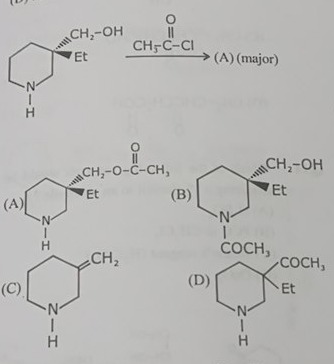
A
(A)
B
(B)
C
(C)
D
(D)
Answer
B
Explanation
Solution
The reaction involves acylation with acetyl chloride (CH3COCl). Both the amine and alcohol groups are nucleophilic. However, amines are generally more nucleophilic than alcohols due to the lower electronegativity of nitrogen compared to oxygen. Therefore, the amine group is preferentially acylated, forming an amide.
The reaction of the amine: R2NH+CH3COCl→R2N-COCH3+HCl
The reaction of the alcohol: ROH+CH3COCl→R-OCOCH3+HCl
Since the amine is more nucleophilic, it reacts faster, leading to the amide formation as the major product. Option (B) shows the acylation of the amine group.
