Question
Question: ```text + CO $\xrightarrow{1. AlCl_3}$A$\xrightarrow{Zn-Hg, HCl, heat}$B$\xrightarrow{1. SOCl_2 \...
+ CO $\xrightarrow{1. AlCl_3}$A$\xrightarrow{Zn-Hg, HCl, heat}$B$\xrightarrow{1. SOCl_2 \atop 2. AlCl_3 \atop 3. H_3O^+}$C. CO $\qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad \qquad$ 2.H3O+
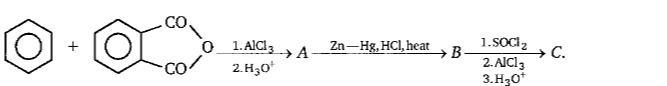
Answer
fluorenone
Explanation
Solution
Explanation
- Step A (Friedel–Crafts acylation): Benzene reacts with the cyclic anhydride (phthalic anhydride) in the presence of AlCl₃/H₃O⁺. This gives an acylated benzoic acid derivative (o-benzoylbenzoic acid).
- Step B (Clemmensen reduction): Zn–Hg/HCl with heat selectively reduces the keto group (–CO–) in the acyl substituent to –CH₂–. The product is now 2‑phenylbenzoic acid.
- Step C (Cyclization via Friedel–Crafts acylation): Treatment with SOCl₂ converts the carboxylic acid group into the corresponding acid chloride. In the presence of AlCl₃ and H₃O⁺, an intramolecular Friedel–Crafts acylation takes place to form a new ring, yielding the tricyclic ketone known as fluorenone.
Answer
The final product is fluorenone.
