Question
Question: Which statement(s) is/are correct :...
Which statement(s) is/are correct :
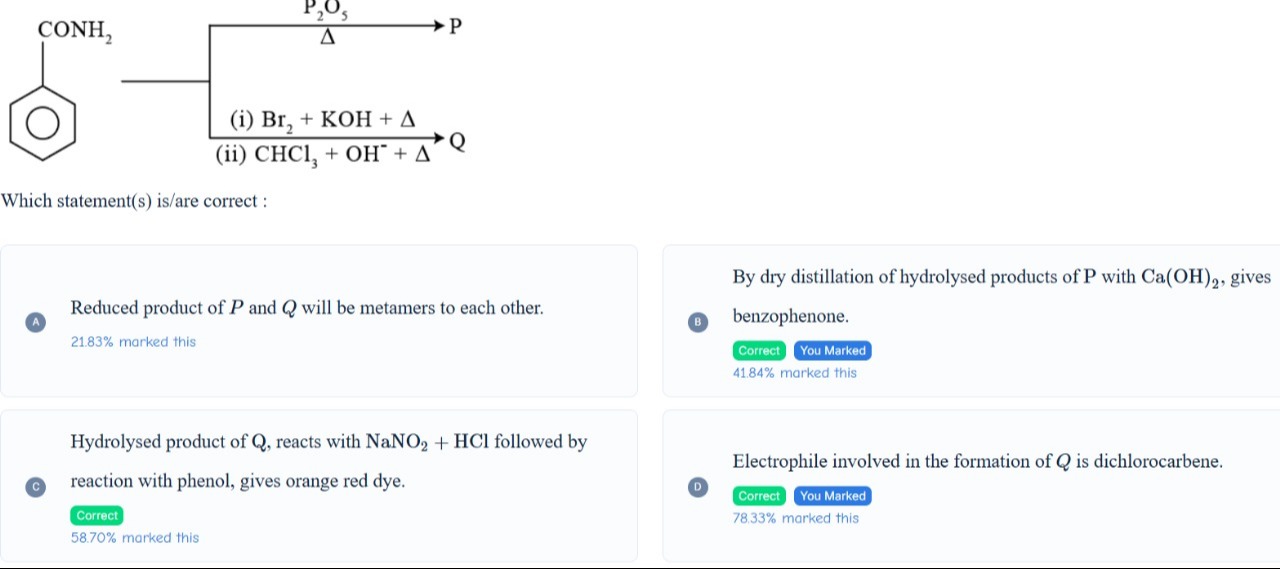
Reduced product of P and Q will be metamers to each other.
By dry distillation of hydrolysed products of P with Ca(OH)2, gives benzophenone.
Hydrolysed product of Q, reacts with NaNO2 + HCl followed by reaction with phenol, gives orange red dye.
Electrophile involved in the formation of Q is dichlorocarbene.
B, C, D
Solution
The starting material is benzamide (C6H5CONH2).
Synthesis of P: Benzamide is treated with P2O5 and heat. P2O5 is a dehydrating agent. Dehydration of benzamide gives benzonitrile. C6H5CONH2P2O5,ΔC6H5CN So, P is benzonitrile.
Synthesis of Q: Benzamide is treated with (i) Br2+KOH+Δ and (ii) CHCl3+OH−+Δ. Step (i) is Hoffmann bromamide degradation, which converts benzamide to aniline. C6H5CONH2Br2,KOH,ΔC6H5NH2 Step (ii) is Carbylamine reaction (Hofmann isocyanide synthesis), where a primary amine (aniline) reacts with chloroform and a strong base to form an isocyanide. C6H5NH2+CHCl3+3OH−ΔC6H5NC+3Cl−+3H2O So, Q is phenyl isocyanide.
Now, let's evaluate the statements:
Statement A: Reduced product of P and Q will be metamers to each other. Reduced product of P (benzonitrile): C6H5CNReductionC6H5CH2NH2 (Benzylamine, a primary amine). Reduced product of Q (phenyl isocyanide): C6H5NCReductionC6H5NHCH3 (N-methylaniline, a secondary amine). Benzylamine and N-methylaniline have the same molecular formula C7H9N. However, they are functional isomers (primary amine vs secondary amine), not metamers. Metamers are isomers with the same molecular formula and the same functional group but different alkyl groups around the functional group. Thus, statement A is incorrect.
Statement B: By dry distillation of hydrolysed products of P with Ca(OH)2, gives benzophenone. Hydrolysed product of P (benzonitrile) is benzoic acid (C6H5COOH). Dry distillation of calcium salt of benzoic acid (calcium benzoate) gives benzophenone. 2C6H5COOH+Ca(OH)2→(C6H5COO)2Ca+2H2O (C6H5COO)2CaDry distillation(C6H5)2CO+CaCO3 So, dry distillation of hydrolysed product of P with Ca(OH)2 (implying formation of calcium salt and then dry distillation) gives benzophenone. Thus, statement B is correct.
Statement C: Hydrolysed product of Q, reacts with NaNO2+HCl followed by reaction with phenol, gives orange red dye. Hydrolysis of Q (phenyl isocyanide) gives aniline. C6H5NC+2H2OH+C6H5NH2+HCOOH Aniline reacts with NaNO2+HCl (nitrous acid) at 0-5 ∘C to form benzenediazonium chloride (diazotization). C6H5NH2+NaNO2+2HCl0−5∘CC6H5N2+Cl−+NaCl+2H2O Benzenediazonium chloride undergoes azo coupling with phenol in alkaline medium to form p-hydroxyazobenzene, which is an orange-red dye. C6H5N2+Cl−+C6H5OHOH−C6H5-N=N-C6H4-OH (para)+HCl Thus, statement C is correct.
Statement D: Electrophile involved in the formation of Q is dichlorocarbene. Q is formed in the Carbylamine reaction. The Carbylamine reaction involves the generation of dichlorocarbene (:CCl2) from chloroform and a strong base, which acts as the electrophile that attacks the primary amine. CHCl3+OH−⇌CCl3−+H2O CCl3−→:CCl2+Cl− Thus, the electrophile involved in the formation of Q is dichlorocarbene. Thus, statement D is correct.
Statements B, C, and D are correct.
