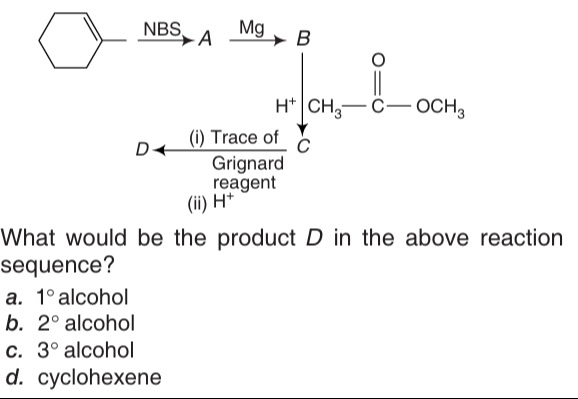
Question
Question: What would be the product D in the above reaction sequence?...
What would be the product D in the above reaction sequence?
1° alcohol
2° alcohol
3° alcohol
cyclohexene
3° alcohol
Solution
The reaction sequence is as follows:
-
Cyclohexene reacts with NBS (N-bromosuccinimide). NBS is a reagent for allylic bromination. In cyclohexene, the allylic positions are the carbons adjacent to the double bond. Both allylic positions are equivalent due to symmetry. So, allylic bromination occurs. Cyclohexene + NBS -> 3-bromocyclohexene (A)
-
3-bromocyclohexene (A) reacts with Mg. This is the formation of a Grignard reagent. 3-bromocyclohexene + Mg -> Cyclohex-2-en-1-ylmagnesium bromide (B)
-
Product B (Cyclohex-2-en-1-ylmagnesium bromide) reacts with methyl acetate (CH3COOCH3) followed by treatment with H+ to form C. The reaction of a Grignard reagent with an ester involves the addition of two equivalents of the Grignard reagent to the ester, followed by hydrolysis, to form a tertiary alcohol.
First addition: Cyclohex-2-en-1-ylMgBr adds to the carbonyl carbon of methyl acetate. A tetrahedral intermediate is formed, which then eliminates the methoxide group to form a ketone.
CH3COOCH3 + Cyclohex-2-en-1-ylMgBr -> CH3-C(−OMgBr)(OCH3)-Cyclohex-2-en-1-yl -> CH3-C(=O)-Cyclohex-2-en-1-yl + MgBr(OCH3)
Second addition: The ketone reacts with another equivalent of the Grignard reagent.
CH3-C(=O)-Cyclohex-2-en-1-yl + Cyclohex-2-en-1-ylMgBr -> CH3-C(−OMgBr)(Cyclohex-2-en-1-yl)-Cyclohex-2-en-1-yl
Hydrolysis: Treatment with H+ hydrolyzes the alkoxide to a tertiary alcohol.
CH3-C(−OMgBr)(Cyclohex-2-en-1-yl)-Cyclohex-2-en-1-yl + H+ -> CH3-C(−OH)(Cyclohex-2-en-1-yl)-Cyclohex-2-en-1-yl (C)
So, C is a tertiary alcohol where the carbon bearing the hydroxyl group is bonded to a methyl group and two cyclohex-2-en-1-yl groups.
-
Product C is treated with (i) Trace of Grignard reagent (B) and (ii) H+ to form D. If C is a tertiary alcohol, it has an acidic proton on the hydroxyl group. A Grignard reagent is a strong base and will react with this acidic proton, forming an alkoxide.
CH3-C(−OH)(R)-R + R'MgBr -> CH3-C(−O−)R-RMgBr+ + R'H (where R = Cyclohex-2-en-1-yl and R' = Cyclohex-2-en-1-yl)
Since only a trace of Grignard reagent is added, it will deprotonate some of the alcohol molecules. Subsequent treatment with H+ will protonate the alkoxide back to the alcohol.
CH3-C(−O−)R-RMgBr+ + H+ -> CH3-C(−OH)R-R + MgBr+
Thus, the reaction of the tertiary alcohol C with a trace of Grignard reagent followed by acid workup will essentially result in the recovery of the tertiary alcohol C. Therefore, D = C.
Product D is the tertiary alcohol (Cyclohex-2-en-1-yl)2-C(−OH)CH3. This is a tertiary alcohol.
Let's consider the possibility that C is the ketone CH3-C(=O)-Cyclohex-2-en-1-yl. Then reaction with a trace of Grignard reagent B followed by H+ would lead to the tertiary alcohol formed by the addition of one equivalent of B to the ketone. This is the same tertiary alcohol as C obtained when two equivalents of B react with the ester.
Based on the typical reaction of Grignard reagents with esters, C is the tertiary alcohol. The reaction from C to D with a trace of Grignard reagent and acid workup leads to the same tertiary alcohol. Therefore, D is a tertiary alcohol.
