Question
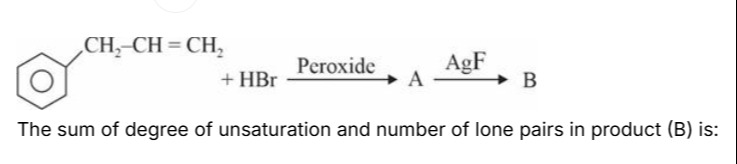
Question: The sum of degree of unsaturation and number of lone pairs in product (B) is:...
The sum of degree of unsaturation and number of lone pairs in product (B) is:
7
Solution
The problem involves a two-step reaction sequence followed by calculations of degree of unsaturation and lone pairs for the final product.
Step 1: Determine Product (A)
The starting material is 3-phenylprop-1-ene (C₆H₅-CH₂-CH=CH₂). It reacts with HBr in the presence of peroxide. This is a free-radical addition reaction, which follows the anti-Markovnikov rule. According to this rule, the bromine atom adds to the less substituted carbon of the double bond, and the hydrogen atom adds to the more substituted carbon.
The double bond is between C1 (terminal CH₂) and C2 (CH).
- C1 is less substituted (primary carbon).
- C2 is more substituted (secondary carbon, attached to C1 and C3).
Therefore, Br adds to C1 and H adds to C2.
The product (A) is 1-bromo-3-phenylpropane.
Structure of (A): C₆H₅-CH₂-CH₂-CH₂Br
Step 2: Determine Product (B)
Product (A) (1-bromo-3-phenylpropane) reacts with AgF. This is a halogen exchange reaction, specifically a variant of the Swarts reaction, used to synthesize fluoroalkanes from bromoalkanes. The bromine atom is replaced by a fluorine atom.
The product (B) is 1-fluoro-3-phenylpropane.
Structure of (B): C₆H₅-CH₂-CH₂-CH₂F
The chemical formula of product (B) is C₉H₁₁F.
Step 3: Calculate the Degree of Unsaturation (DU) for Product (B)
Product (B) is C₉H₁₁F. The formula for calculating the Degree of Unsaturation (DU) is:
DU = C + 1 - (H/2) - (X/2) + (N/2)
Where:
C = number of carbon atoms = 9 H = number of hydrogen atoms = 11 X = number of halogen atoms (F, Cl, Br, I) = 1 (for F) N = number of nitrogen atoms = 0
Substitute the values into the formula:
DU = 9 + 1 - (11/2) - (1/2) + (0/2)
DU = 10 - 5.5 - 0.5 + 0
DU = 10 - 6
DU = 4
Alternatively, by structure: The benzene ring contributes 4 to the DU (3 double bonds + 1 ring). The alkyl chain is saturated and does not add to the DU. So, DU = 4.
Step 4: Count the Number of Lone Pairs in Product (B)
Product (B) is C₆H₅-CH₂-CH₂-CH₂F.
- Carbon atoms (C) in stable organic compounds form four bonds and typically have no lone pairs.
- Hydrogen atoms (H) form one bond and have no lone pairs.
- Fluorine atom (F) is a halogen from Group 17. It forms one covalent bond and has three lone pairs (6 non-bonding electrons) to complete its octet.
Therefore, the total number of lone pairs in product (B) is 3 (all contributed by the fluorine atom).
Step 5: Calculate the Sum of DU and Number of Lone Pairs
Sum = Degree of Unsaturation + Number of Lone Pairs Sum = 4 + 3 Sum = 7
The final answer is 7
Explanation of the Solution:
- Reaction 1 (HBr/Peroxide): 3-phenylprop-1-ene undergoes anti-Markovnikov addition of HBr due to the presence of peroxide, yielding 1-bromo-3-phenylpropane (Product A).
- Reaction 2 (AgF): Product A undergoes halogen exchange with AgF (Swarts reaction), replacing bromine with fluorine to form 1-fluoro-3-phenylpropane (Product B).
- Degree of Unsaturation (DU): For C₉H₁₁F (Product B), DU = C + 1 - H/2 - X/2 = 9 + 1 - 11/2 - 1/2 = 10 - 5.5 - 0.5 = 4. This corresponds to the benzene ring.
- Lone Pairs: In C₉H₁₁F, carbon and hydrogen atoms have no lone pairs. The fluorine atom has 3 lone pairs.
- Sum: Total DU (4) + Total lone pairs (3) = 7.
Answer:
The sum of degree of unsaturation and number of lone pairs in product (B) is 7.
