Question
Question: The reagent X may be...
The reagent X may be
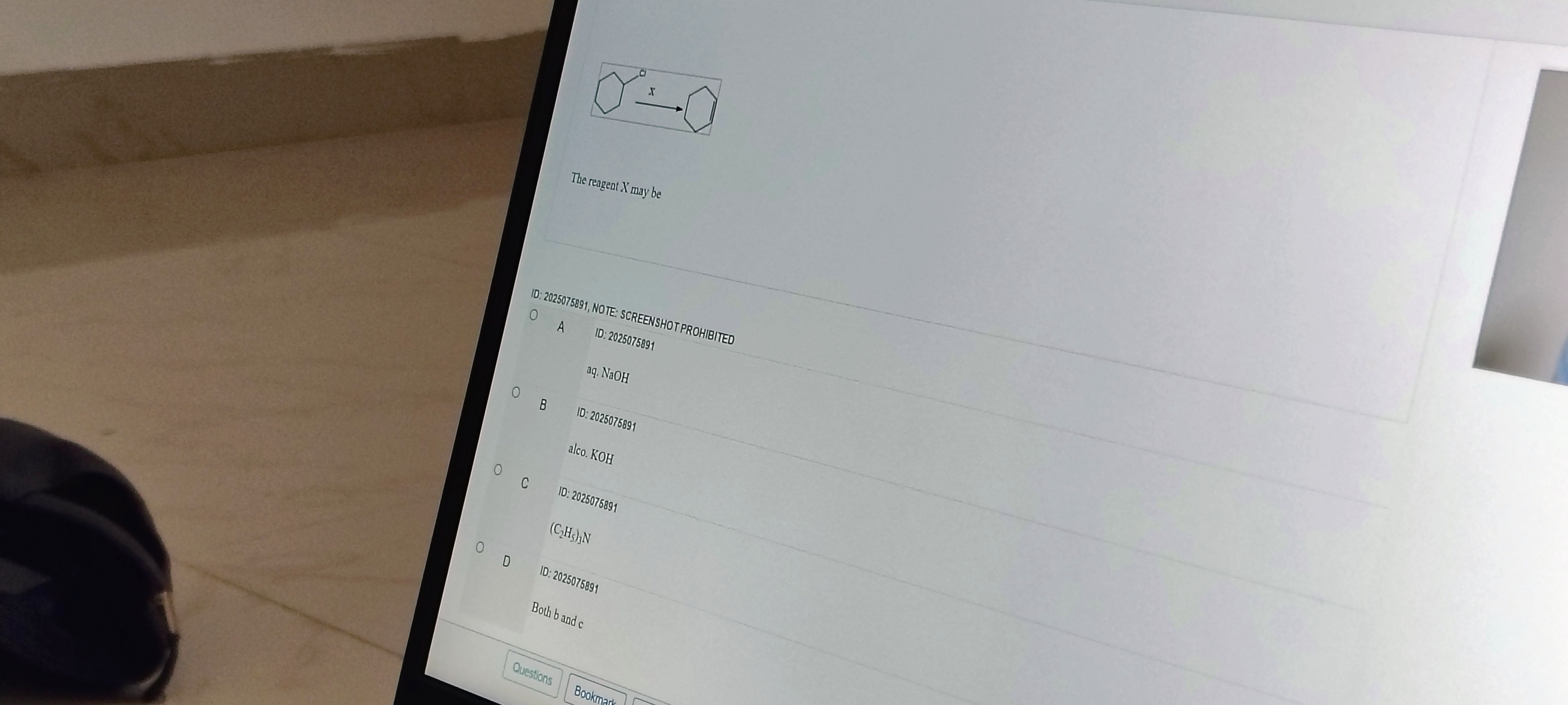
aq. NaOH
alco. KOH
(C2H5)3N
Both b and c
D
Solution
The reaction shown is the conversion of cyclohexyl chloride to cyclohexene. This is a dehydrohalogenation reaction, which is a type of elimination reaction where a hydrogen atom and a halogen atom are removed from adjacent carbon atoms, leading to the formation of a double bond (alkene).
Let's analyze the given options for reagent X:
-
A. aq. NaOH (Aqueous Sodium Hydroxide): Aqueous NaOH is a strong base and a strong nucleophile. When a haloalkane reacts with aqueous NaOH, the primary reaction pathway is typically nucleophilic substitution (SN1 or SN2), leading to the formation of an alcohol. For cyclohexyl chloride (a secondary haloalkane), it would primarily yield cyclohexanol. Therefore, aqueous NaOH is not the correct reagent for forming cyclohexene.
-
B. alco. KOH (Alcoholic Potassium Hydroxide): Alcoholic KOH is a strong base that favors elimination reactions over substitution reactions. The hydroxide ion (OH−) in an alcoholic solvent acts as a strong base, abstracting a proton from a β-carbon (carbon adjacent to the carbon bearing the halogen) and simultaneously eliminating the halogen, forming an alkene. This is a classic method for dehydrohalogenation.
Reaction:
\ce{C6H11Cl + KOH (alc.) -> C6H10 + KCl + H2O}
- C. (C2H5)3N (Triethylamine): Triethylamine is a tertiary amine and a relatively strong, non-nucleophilic base. Due to its bulky nature and the absence of a hydrogen atom on the nitrogen for nucleophilic attack (like in primary or secondary amines), it acts primarily as a base and favors elimination reactions (specifically E2). It can abstract a β-hydrogen, leading to the elimination of HCl and the formation of cyclohexene.
Reaction:
\ce{C6H11Cl + (C2H5)3N -> C6H10 + (C2H5)3NH+Cl-}
- D. Both b and c: Since both alcoholic KOH and triethylamine are strong bases capable of promoting dehydrohalogenation reactions to convert cyclohexyl chloride into cyclohexene, both are suitable reagents for X.
The final answer is D
Explanation of the solution: The transformation from cyclohexyl chloride to cyclohexene is a dehydrohalogenation (elimination of HCl). This reaction requires a strong base.
- Aqueous NaOH primarily promotes nucleophilic substitution, yielding cyclohexanol.
- Alcoholic KOH is a strong base commonly used for dehydrohalogenation, forming alkenes.
- Triethylamine is a strong, non-nucleophilic base that also promotes elimination reactions (E2) over substitution.
Therefore, both alcoholic KOH and triethylamine can serve as reagent X.
