Question
Question: The above apparatus consists of three temperature jacketed 12.315 litre bulbs connected by stop cock...
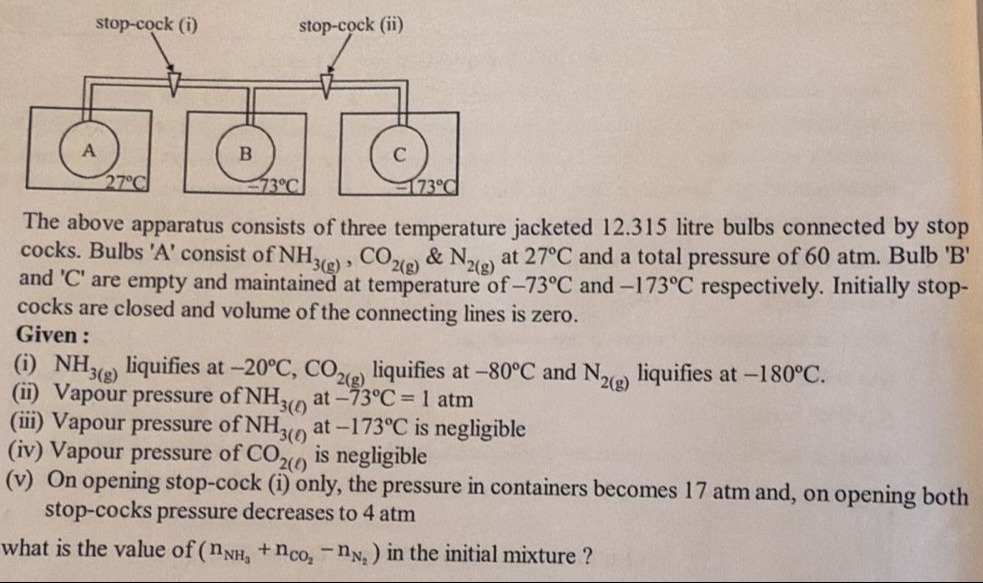
The above apparatus consists of three temperature jacketed 12.315 litre bulbs connected by stop cocks. Bulbs 'A' consist of NH3(g), CO2(g) & N2(g) at 27°C and a total pressure of 60 atm. Bulb 'B' and 'C' are empty and maintained at temperature of −73°C and −173°C respectively. Initially stop-cocks are closed and volume of the connecting lines is zero.
Given:
(i) NH3(g) liquifies at −20°C, CO2(g) liquifies at −80°C and N2(g) liquifies at −180°C.
(ii) Vapour pressure of NH3(l) at −73°C = 1 atm
(iii) Vapour pressure of NH3(l) at −173°C is negligible
(iv) Vapour pressure of CO2(l) is negligible
(v) On opening stop-cock (i) only, the pressure in containers becomes 17 atm and, on opening both stop-cocks pressure decreases to 4 atm
what is the value of (nNH3+nCO2−nN2) in the initial mixture ?
8
Solution
Let V=12.315 litre be the volume of each bulb.
Initial state: Bulb A has NH3, CO2, N2 at TA=27∘C=300 K and total pressure PA=60 atm. Bulb B is at TB=−73∘C=200 K, Bulb C is at TC=−173∘C=100 K. Bulbs B and C are initially empty.
Let nNH3, nCO2, nN2 be the initial moles of NH3, CO2, and N2 in bulb A. Initial total moles in bulb A: nA=nNH3+nCO2+nN2. Using the ideal gas law for bulb A initially: PAV=nARTA. 60×12.315=nAR×300. nAR=30060×12.315=512.315=2.463. So, nNH3R+nCO2R+nN2R=2.463.
Case 1: Stop-cock (i) is opened. Bulbs A (300 K) and B (200 K) are connected. The total volume is VA+VB=2V. Liquefaction points: NH3 at 253 K, CO2 at 193 K, N2 at 93 K. At TB=200 K: NH3 (liquefies at 253 K): 200<253, so NH3 can condense. Vapour pressure of NH3(l) at 200 K is 1 atm. CO2 (liquefies at 193 K): 200>193, so CO2 remains as gas. Vapour pressure of CO2(l) is negligible (meaning if liquid is present, gas pressure is negligible), but at 200 K it's a gas. N2 (liquefies at 93 K): 200>93, so N2 remains as gas.
After opening stop-cock (i), the pressure in containers becomes P1=17 atm. In bulb A (300 K), all gases are in gaseous state. In bulb B (200 K), CO2 and N2 are in gaseous state. NH3 can be in gaseous and liquid state. Let PNH3′, PCO2′, PN2′ be the partial pressures of the gases in the gaseous phase. The total pressure is P1=PNH3′+PCO2′+PN2′=17. If NH3 condenses in bulb B, its partial pressure in the gas phase is equal to the vapour pressure at 200 K, which is 1 atm. So PNH3′=1 atm. Then PCO2′+PN2′=17−1=16 atm.
Assuming PNH3′=1 atm. Total moles of NH3: nNH3=nNH3,A′+nNH3,B′+nNH3,liquid′. nNH3,A′=RTAPNH3′V=R×3001×V. nNH3,B′=RTBPNH3′V=R×2001×V. Moles of NH3 in gas phase after opening (i): nNH3,gas′=nNH3,A′+nNH3,B′=RV(3001+2001)=RV6005=120RV. Total initial moles of NH3 is nNH3=RTAPNH3,AV. If PNH3,A>120TA=120300=2.5, then condensation occurs.
Total moles of CO2: nCO2=nCO2,A′+nCO2,B′=RTAPCO2′V+RTBPCO2′V=RPCO2′V(3001+2001)=120RPCO2′V. Total moles of N2: nN2=nN2,A′+nN2,B′=RTAPN2′V+RTBPN2′V=RPN2′V(3001+2001)=120RPN2′V.
From the initial state, nCO2=RTAPCO2,AV=300RPCO2,AV. So, 300RPCO2,AV=120RPCO2′V⟹PCO2,A=120300PCO2′=2.5PCO2′. Similarly, PN2,A=2.5PN2′. PCO2,A+PN2,A=2.5(PCO2′+PN2′)=2.5×16=40 atm. Initial partial pressure of NH3 in A: PNH3,A=60−(PCO2,A+PN2,A)=60−40=20 atm. Since PNH3,A=20>2.5, condensation of NH3 occurs in bulb B, so our assumption PNH3′=1 atm is correct.
Initial moles of each gas: nNH3=RTAPNH3,AV=300R20V=15RV. nCO2=RTAPCO2,AV=300R2.5PCO2′V=120RPCO2′V. nN2=RTAPN2,AV=300R2.5PN2′V=120RPN2′V. nCO2+nN2=120R(PCO2′+PN2′)V=120R16V=15R2V.
Check total initial moles: nA=nNH3+nCO2+nN2=15RV+15R2V=15R3V=5RV. From 60V=nARTA=nAR×300, nAR=30060V=5V. So nA=5RV. This matches.
Case 2: Both stop-cocks (i) and (ii) are opened. Bulbs A (300 K), B (200 K), and C (100 K) are connected. The total volume is VA+VB+VC=3V. The pressure decreases to P2=4 atm. At TC=100 K: NH3 (liquefies at 253 K): 100<253, can condense. Vapour pressure of NH3(l) at 100 K is negligible. CO2 (liquefies at 193 K): 100<193, can condense. Vapour pressure of CO2(l) is negligible. N2 (liquefies at 93 K): 100>93, remains as gas.
In the final state, the partial pressures of NH3 and CO2 in the gas phase are negligible (close to 0) if liquid is present. Since the total pressure is 4 atm, and the partial pressures of NH3 and CO2 are negligible, the partial pressure of N2 must be approximately 4 atm. Let PNH3′′, PCO2′′, PN2′′ be the partial pressures in the gas phase. PNH3′′+PCO2′′+PN2′′=4. Given that vapour pressure of NH3(l) at 100 K is negligible, PNH3′′≈0. Given that vapour pressure of CO2(l) is negligible, PCO2′′≈0. So, PN2′′≈4 atm.
Let's check if N2 remains as gas. The partial pressure of N2 is 4 atm. The temperature is 100 K. The liquefaction point is 93 K. Since the lowest temperature is 100 K > 93 K, N2 remains as gas in all bulbs.
Total moles of N2: nN2=nN2,A′′+nN2,B′′+nN2,C′′. nN2,A′′=RTAPN2′′V=R×3004V. nN2,B′′=RTBPN2′′V=R×2004V. nN2,C′′=RTCPN2′′V=R×1004V. nN2=R4V(3001+2001+1001)=R4V(6002+3+6)=R4V60011=600R44V=150R11V.
We had nN2=300R2.5PN2′V=120RPN2′V. So, 120RPN2′V=150R11V⟹PN2′=15011×120=1511×12=511×4=8.8 atm. Then PCO2′=16−PN2′=16−8.8=7.2 atm.
Now we can calculate the initial moles. nNH3=15RV. nCO2=120RPCO2′V=120R7.2V=1200R72V=100R6V=50R3V. nN2=120RPN2′V=120R8.8V=1200R88V=300R22V=150R11V.
We need to find the value of (nNH3+nCO2−nN2). (nNH3+nCO2−nN2)=15RV+50R3V−150R11V. Find a common denominator, which is 150. =150R10V+150R9V−150R11V=150R(10+9−11)V=150R8V=75R4V.
We know nAR=2.463, so R=nA2.463. Also nA=5RV. R=(5RV)2.463=V2.463×5R. 1=V2.463×5⟹V=12.315. This confirms the value of V. R=nA2.463. We also have nA=5RV.
Let's express the result in terms of V/R. RV=R12.315. Using R=0.0821, V/R=12.315/0.0821≈150. Using R=0.082, V/R=12.315/0.082≈150.18. Let's use V/R=150 as a round number implied by the problem. nNH3=15150=10. nCO2=503×150=3×3=9. nN2=15011×150=11. Check total moles: 10+9+11=30. nA=5RV=5150=30. This matches.
The value of (nNH3+nCO2−nN2)=10+9−11=19−11=8.
Final check of assumptions: In Case 1, PNH3′=1. nNH3,gas′=120R1×V=120150=1.25. Initial moles nNH3=10. Since 10>1.25, condensation occurs in B. In Case 2, PNH3′′=0. nNH3,gas′′=R0×V(3001+2001+1001)=0. This implies all NH3 is condensed. Total moles of NH3 is 10. This is possible if the partial pressure is negligible. In Case 2, PCO2′′=0. nCO2,gas′′=0. This implies all CO2 is condensed. Total moles of CO2 is 9. This is possible if the partial pressure is negligible. In Case 2, PN2′′=4. nN2,gas′′=150R4V=1504×150=4. Total moles of N2 is 11. So, 11−4=7 moles of N2 are not in the gas phase. This is a contradiction, as N2 should remain as gas at 100 K.
Let's re-evaluate the partial pressure of N2 in Case 2. Total moles of N2 is nN2=11. After opening both stop-cocks, N2 is distributed in A, B, and C as gas. nN2=nN2,A′′+nN2,B′′+nN2,C′′=RTAPN2′′V+RTBPN2′′V+RTCPN2′′V. 11=RPN2′′V(3001+2001+1001)=RPN2′′V60011. 11=PN2′′RV60011=PN2′′×150×60011=PN2′′×411. PN2′′=4 atm. This is consistent with the total pressure being 4 atm if NH3 and CO2 partial pressures are negligible.
Let's re-evaluate the partial pressure of NH3 in Case 2. Total moles of NH3 is nNH3=10. Partial pressure of NH3 in gas phase is PNH3′′. Moles of NH3 in gas phase: nNH3,gas′′=RPNH3′′V(3001+2001+1001)=RPNH3′′V60011. nNH3,gas′′=PNH3′′×150×60011=PNH3′′×411. Given vapour pressure of NH3(l) at 100 K is negligible, PNH3′′ should be negligible if liquid is present. If PNH3′′≈0, then nNH3,gas′′≈0. This means almost all NH3 is condensed. This is consistent with nNH3=10.
Let's re-evaluate the partial pressure of CO2 in Case 2. Total moles of CO2 is nCO2=9. Partial pressure of CO2 in gas phase is PCO2′′. Moles of CO2 in gas phase: nCO2,gas′′=RPCO2′′V(3001+2001+1001)=RPCO2′′V60011=PCO2′′×411. Given vapour pressure of CO2(l) is negligible, PCO2′′ should be negligible if liquid is present. If PCO2′′≈0, then nCO2,gas′′≈0. This means almost all CO2 is condensed. This is consistent with nCO2=9.
So, the assumption that partial pressures of NH3 and CO2 are negligible in Case 2 is consistent with the calculated initial moles.
The value of (nNH3+nCO2−nN2)=10+9−11=8.
The final answer is 8.
Explanation:
- Calculate the initial total moles of gas in bulb A using the ideal gas law.
- Analyze the state of each gas when stop-cock (i) is opened, considering liquefaction points and vapour pressures at the given temperatures. Determine which gases remain as gas and which may condense.
- Use the given pressure after opening stop-cock (i) and the conditions in bulbs A and B to set up equations relating the initial moles and partial pressures. Assume condensation of NH3 occurs and verify this assumption.
- Analyze the state of each gas when both stop-cocks are opened, considering liquefaction points and vapour pressures at the given temperatures. Determine which gases remain as gas and which condense.
- Use the given pressure after opening both stop-cocks and the conditions in bulbs A, B, and C to set up equations relating the initial moles and partial pressures. Assume condensation of NH3 and CO2 occurs and verify this assumption.
- Solve the system of equations to find the initial moles of each gas.
- Calculate the required value (nNH3+nCO2−nN2).
The final answer is 8.
