Question
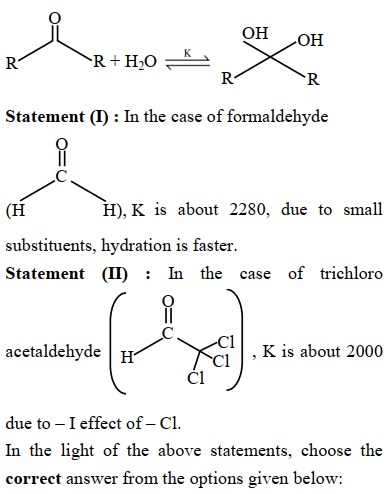
Question: R R + H2O R OH OH R **Statement (I):** In the case of formaldehyde C (H H), K is about 2280, due ...
R R + H2O R OH OH R
Statement (I): In the case of formaldehyde
C (H H), K is about 2280, due to small substituents, hydration is faster.
Statement (II): In the case of trichloro acetaldehyde
C Cl acetaldehyde H K is about 2000 due to – I effect of – Cl. Cl Cl
In the light of the above statements, choose the correct answer from the options given below:
Both Statement (I) and Statement (II) are correct
Both Statement (I) and Statement (II) are incorrect
Statement (I) is correct but Statement (II) is incorrect
Statement (I) is incorrect but Statement (II) is correct
Statement (I) is correct but Statement (II) is incorrect
Solution
The hydration of carbonyl compounds is influenced by the size of substituents and the electron‐withdrawing (–I) effect. In formaldehyde, the small substituents allow rapid and favorable hydration (K ≈ 2280). In contrast, for trichloroacetaldehyde, the strong –I effect of the –Cl groups increases the electrophilicity of the carbonyl carbon so that hydration is extremely favored. Therefore, the reported value of K ≈ 2000 for trichloroacetaldehyde is too low.
