Question
Question: Figure shows two spherical containers P and Q connected by tap T. P contains an ideal gas at pressur...
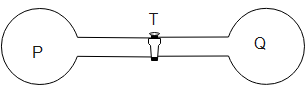
Figure shows two spherical containers P and Q connected by tap T. P contains an ideal gas at pressure 5×105 Nm−2 and temperature 300K . Q contains the same gas at pressure 2×105 Nm−2 and temperature 400K . If the tap is opened, the final pressure becomes m×105 Nm−2 . What is the value of m if the volume of Q is four times the volume of P.
(A) 1.4
(B) 1.8
(C) 2.4
(D) 2.8
Solution
To answer this question, you must recall the ideal gas equation or the equation of state. The ideal gas equation relates all the properties of gas, namely pressure, volume, temperature and number of moles.
PV=nRT
Where, P is the pressure exerted by the gas on the walls of the container
V is the total volume occupied by the gas or the volume of the container
n is the number of moles of the gas present in the container
And, T is the temperature at which the gas is present in the container.
Complete step by step solution:
In the question, as the tap between two containers containing a gas at different physical properties is opened, there will be change in all the properties. The number of moles, however, remains the same as the system is overall a closed system.
Using the ideal gas equation, we can write the number of moles of a gas as, n=RTPV
The number of moles initially in the system is given as the sum of the number of moles of gas in P and Q.
ni=nP+nQ
Similarly, the final number of moles is also given as the sum of the number of moles of gas in P and Q.
nf=nP+nQ
We know that the number of moles of the gas does not change in a closed system, hence, ni=nf
RTPPPVP+RTQPQVQ=RTPPVP+RTQPVQ
Substituting the values given in the question, we get,
3005×105×VP+4002×105×4VP=300P×V+400P×4V
⇒3005×105+4002×105×4=300P+400P×4
Solving this:
⇒P=1.4×105 N/m2
Thus, the correct answer is A.
Note:
The ideal gas equation has been derived using the gas laws, i.e., the Boyle’s Law, Charles Law, Avogadro’s Law and Gay- Lussac’s Law. Hence an ideal gas is that gas which obeys all the gas laws. Ideal gas equation generally is not applicable at higher pressures and lower temperatures.
