Question
Question: Figure shows a vessel partitioned by a fixed diathermic separator. Different ideal gases are filled ...
Figure shows a vessel partitioned by a fixed diathermic separator. Different ideal gases are filled in the two parts. The rms speed of the molecules in the left part equals the mean speed of the molecules in the right part. Calculate the ratio of the mass of a molecule in the left part to the mass of molecule in the right part.
Solution
According to kinetic theory of gases, the gaseous molecules are in constant random motion when the temperature above absolute zero is considered. Depending upon the nature of the molecules’ relative kinetic energies, a collision among the gaseous molecules lead to transfer of kinetic energies and a change in direction. These movements are characterized as the velocities of gaseous molecules.
Complete answer:
Average or mean velocity is the arithmetic means of the speed of the molecules of gases whereas rms speed i.e., root mean square speed is the square root of the arithmetic mean of squares of different speed of the individual molecules.
Let the gases in the left and right side of the vessel are x and y respectively. Then the representation of molecules of gases in the vessel will be as follows:
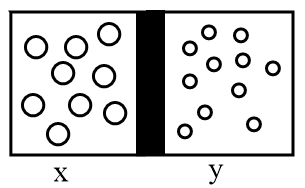
The rms speed of gas x can be calculated as per following expression:
vrms=Mx3RT−(1)
Where, vrms is the rms speed of the gas, R is the universal gas constant and Mx is the mass of the molecule of gas.
The mean i.e., average speed of gas y can be calculated as per following expression:
vmean=πMy8RT−(2)
Where, vmean is the mean speed of the gas, R is the universal gas constant and My is the mass of the molecule of gas.
As per question, the rms speed of molecules of gas x is equal to the mean speed of molecules of gas y. Therefore,
vrms=vmean
Substituting values from equation (1) and (2):
⇒Mx3RT=πMy8RT
On squaring both sides of the equation and cancelling the common terms, the ratio of mass of gas x to the mass of gas y will be as follows:
⇒Mx3RT=πMy8RT
⇒MyMx=83π
Substituting π=3.14:
⇒MyMx=1.18
Hence, the ratio of the mass of a molecule in the left part to the mass of molecule in the right part is 1.18.
Note:
It is important to note that the maximum velocity which can be attained by the gaseous molecules is rms velocity while the minimum velocity which can be attained by maximum fraction of molecules of gases is most probable velocity. The order follows as vrms>vmean>vmps.
