Question
Question: Select the product from the following I: II: CH3-CH-CH2-CH OH III: CH3-CH-CH2-CH OH OH Consider t...
Select the product from the following
I: II: CH3-CH-CH2-CH
OH III: CH3-CH-CH2-CH
OH OH Consider the given
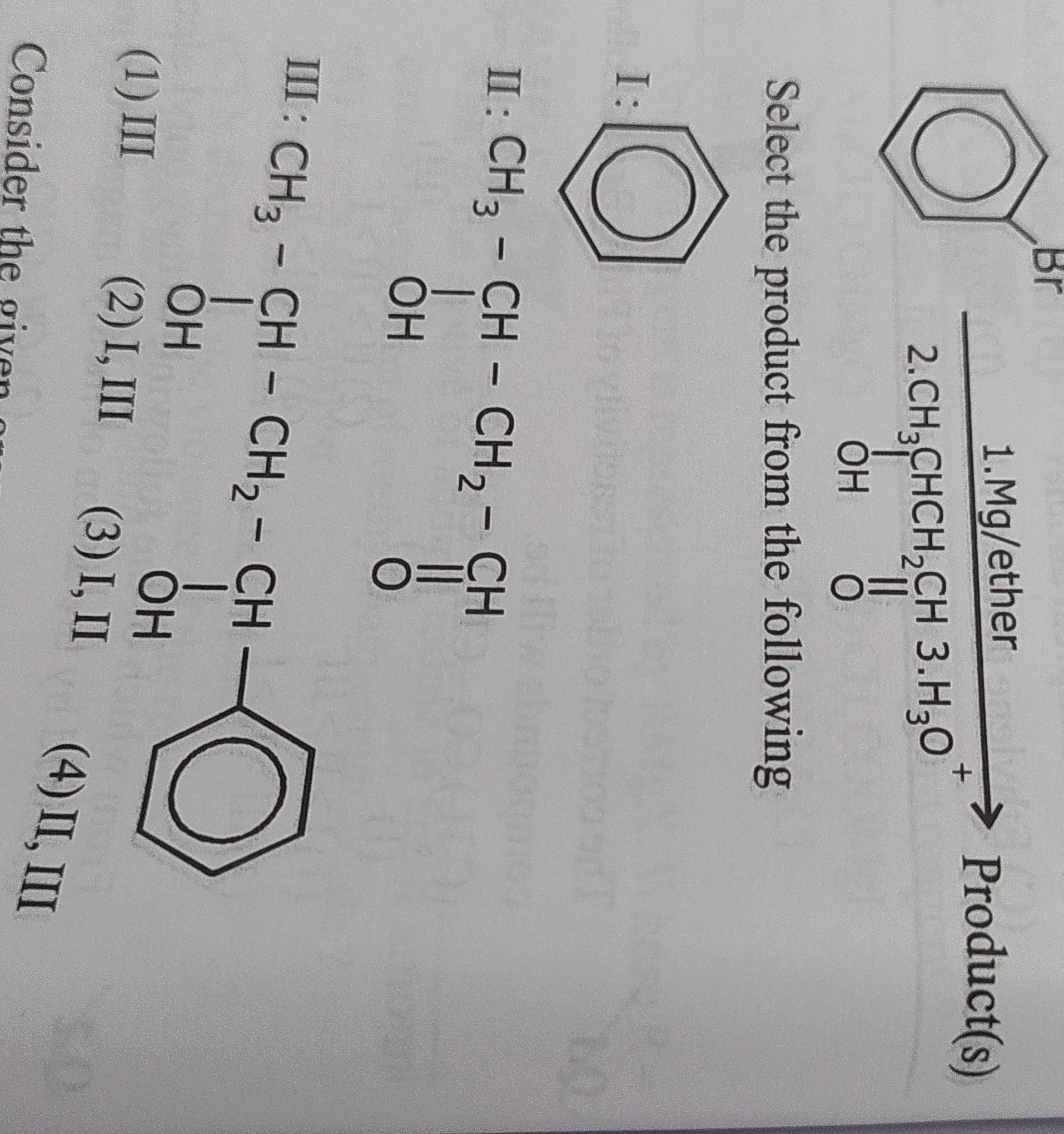
III
I, III
I, II
II, III
(2) I, III
Solution
The reaction involves the Grignard reagent formed from bromobenzene and a hydroxyl-aldehyde compound.
Step 1: Formation of Grignard Reagent
Bromobenzene reacts with magnesium in ether to form phenylmagnesium bromide.
C6H5Br+MgetherC6H5MgBr
Step 2: Reaction of Grignard Reagent with the Organic Substrate
The organic substrate given in the reaction scheme is CH3CH2CH2CH(OH)CHO (4-hydroxypentanal). However, the structures provided in options II and III, particularly III, suggest that the intended reactant might be CH3CH(OH)CH2CHO (3-hydroxybutanal), which has a 4-carbon chain, matching the skeleton of options II and III. Assuming there is a typo in the reactant structure and it should be 3-hydroxybutanal, we proceed with this assumption as it leads to one of the given product options.
The reactant, CH3CH(OH)CH2CHO, contains two functional groups:
- A hydroxyl group (-OH), which has an acidic proton.
- An aldehyde group (-CHO), which is an electrophilic carbon for nucleophilic attack.
Grignard reagents are strong bases and strong nucleophiles.
-
Acid-Base Reaction: Grignard reagents react very rapidly with acidic protons (like those in -OH groups).
C6H5MgBr+CH3CH(OH)CH2CHO→C6H6+CH3CH(OMgBr)CH2CHO
This reaction produces benzene (C6H6), which is Product I. This reaction consumes one equivalent of the Grignard reagent.
-
Nucleophilic Addition: After the acidic proton is removed (or if excess Grignard reagent is used), the Grignard reagent can attack the carbonyl carbon of the aldehyde.
If excess C6H5MgBr is used, after the first equivalent reacts with the -OH group, the remaining Grignard reagent will react with the aldehyde group of the resulting alkoxide:
CH3CH(OMgBr)CH2CHO+C6H5MgBr→CH3CH(OMgBr)CH2CH(OMgBr)C6H5
Step 3: Hydrolysis
The reaction mixture is then hydrolyzed with H3O+. This protonates the alkoxide intermediates back to alcohols.
CH3CH(OMgBr)CH2CH(OMgBr)C6H5H3O+CH3CH(OH)CH2CH(OH)C6H5
This product is 1-phenylbutane-1,3-diol, which matches Product III.
Therefore, if excess Grignard reagent is used, both the acid-base reaction and the nucleophilic addition to the carbonyl will occur, leading to the formation of both benzene (I) and 1-phenylbutane-1,3-diol (III).
Let's re-verify the options based on the structures given:
I: Benzene (C6H6)
II: CH3-CH(OH)-CH2-CHO (3-hydroxybutanal) - This is the reactant (if we assume the typo). It's not a product of the reaction.
III: CH3-CH(OH)-CH2-CH(OH)-C6H5 (1-phenylbutane-1,3-diol) - This is the product of nucleophilic addition.
Since both I and III are formed under typical conditions where Grignard reagent reacts with a molecule containing both an acidic proton and a carbonyl group (assuming excess Grignard reagent), option (2) which states "I, III" is the correct choice.
Explanation of the solution:
- Bromobenzene reacts with Mg/ether to form phenylmagnesium bromide (C6H5MgBr), a Grignard reagent.
- The substrate, assumed to be 3-hydroxybutanal (CH3CH(OH)CH2CHO) due to options, has an acidic -OH group and an aldehyde -CHO group.
- Grignard reagents are strong bases. They first react with the acidic -OH group, producing benzene (C6H6, Product I) and an alkoxide.
- If excess Grignard reagent is used, the remaining Grignard reagent acts as a nucleophile and attacks the electrophilic carbon of the aldehyde group in the alkoxide.
- Subsequent hydrolysis (H3O+) converts the intermediate alkoxides into alcohols, yielding 1-phenylbutane-1,3-diol (CH3CH(OH)CH2CH(OH)C6H5, Product III).
- Therefore, both I and III are the products.
