Question
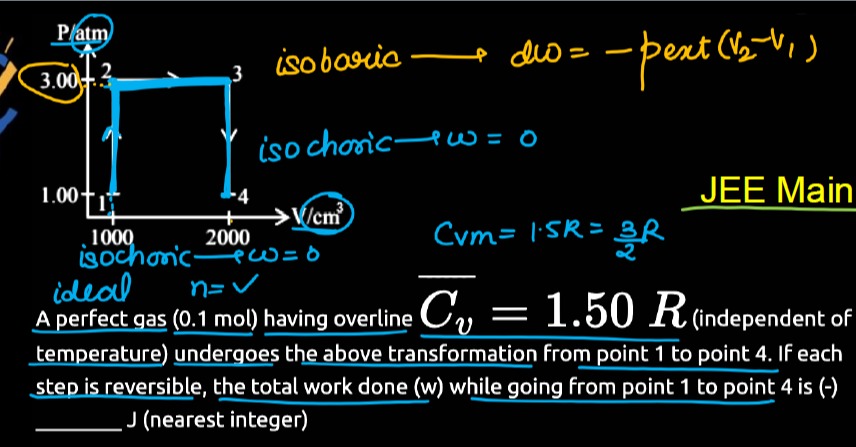
Question: P/atm 3. 00 isobaric $\longrightarrow$ dw= $-P_{ext}(V_{2}-V_{1})$ 1. 00 1 2. 000 V/cm³ isocho...
P/atm
-
00 isobaric ⟶ dw= −Pext(V2−V1)
-
00 1
-
000 V/cm³ isochoric ⟶ w = 0
isochoric ⟶ w = 0
JEE Main
Cvm = 1.5R = 23R
Cv = 1.50 R(independent of temperature) undergoes the above transformation from point 1 to point 4. If each step is reversible, the total work done (w) while going from point 1 to point 4 is (-) J (nearest integer)
304
Solution
The transformation consists of three steps: 1 → 2 (isochoric), 2 → 3 (isobaric), and 3 → 4 (isochoric).
-
Isochoric processes (1 → 2 and 3 → 4): Work done w=0 as there is no change in volume (ΔV=0).
-
Isobaric process (2 → 3):
- Pressure P=3.00 atm.
- Volume change: V2=1000 cm³ = 1 L, V3=2000 cm³ = 2 L.
- Work done w2→3=PΔV=3.00atm×(2L−1L)=3.00L\cdotpatm.
-
Conversion to Joules:
- Using the conversion factor 1L\cdotpatm=101.325J.
- w2→3=3.00×101.325J≈303.975J.
The question asks for the total work done (w) and specifies the answer format as (-)_____ J, indicating work done on the system. The work done by the system during expansion (2 → 3) is positive. Therefore, the work done on the system is negative.
Total work done w=w1→2+w2→3+w3→4=0+(−303.975J)+0≈−304J. The value to fill in the blank is 304.
