Question
Question: Work done by polytropic process formula and area of graph are coming different. why?...
Work done by polytropic process formula and area of graph are coming different. why?
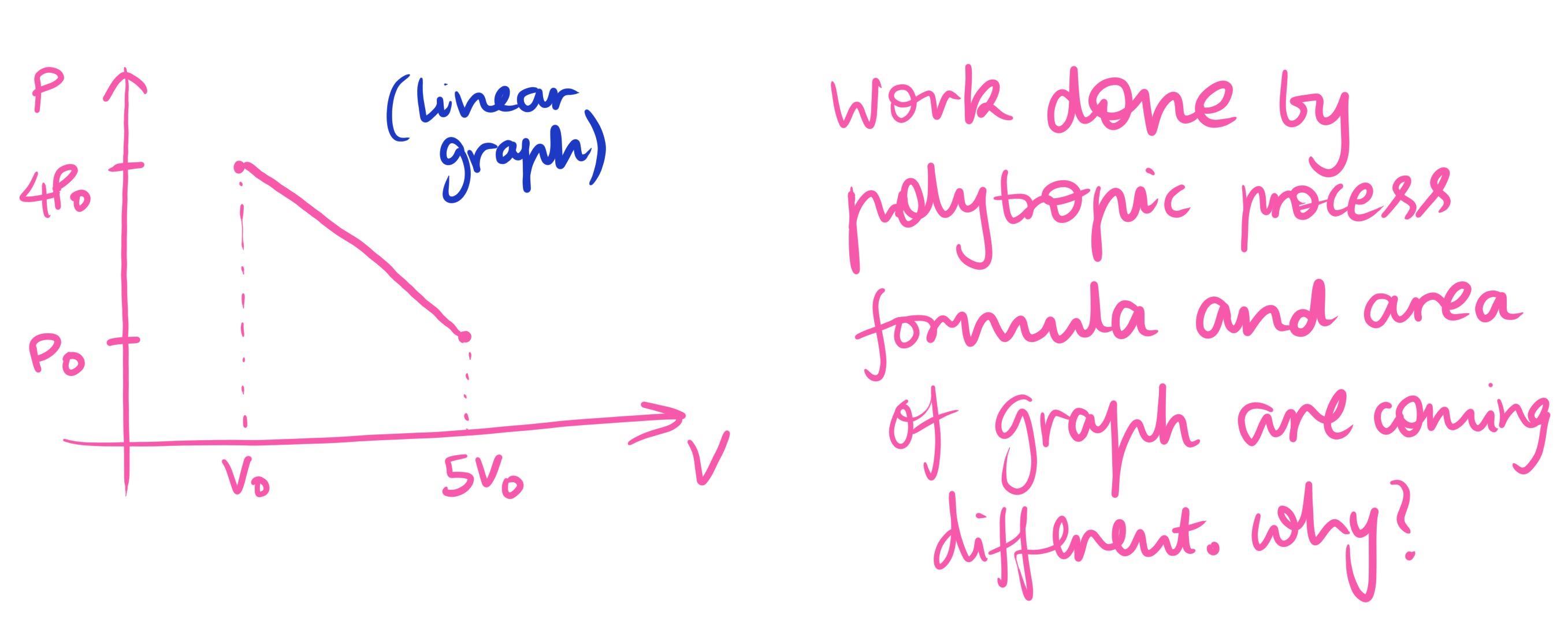
The discrepancy arises because the given process is a linear relationship between pressure and volume, while the polytropic process formula is derived for processes that follow the relationship PVn=constant. A linear process is not generally equivalent to PVn=constant for a single value of n. Therefore, the polytropic formula is not applicable to this linear process, leading to a different result than the actual work done calculated from the area under the graph.
Solution
The given process is a linear relationship between pressure (P) and volume (V). The work done by the gas is represented by the area under the P-V graph. The formula for work done in a polytropic process, W=1−nP2V2−P1V1, is derived under the assumption that the process follows the relation PVn=constant. A linear relationship between P and V (P=mV+c) is generally not equivalent to PVn=constant for any single value of n. Since the given linear process is not a polytropic process of the form PVn=constant, applying the polytropic formula to it yields a result different from the actual work done calculated from the area under the graph.
