Question
Question: Major product Z is:...
Major product Z is:
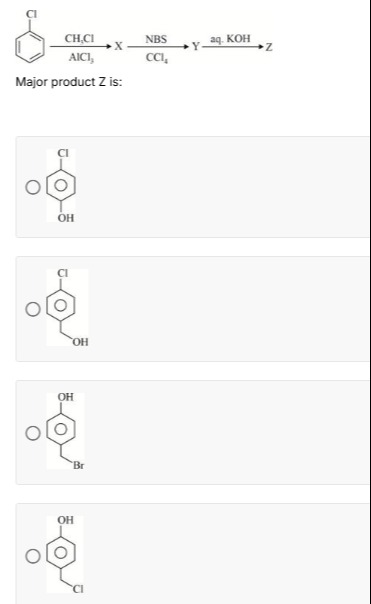
Option 2
Solution
The reaction sequence involves three steps:
Step 1: Friedel-Crafts Alkylation
The starting material is chlorobenzene. Chlorobenzene reacts with methyl chloride (CH₃Cl) in the presence of anhydrous aluminium chloride (AlCl₃). This is a Friedel-Crafts alkylation reaction.
Chlorine (-Cl) is an ortho-para directing group due to the resonance effect (+M effect) but deactivating due to its strong inductive effect (-I effect). In Friedel-Crafts alkylation, the directing effect dominates, and the incoming methyl group (-CH₃) will substitute at the ortho or para positions. Due to steric hindrance, the para product is typically the major product.
Therefore, product X is 1-chloro-4-methylbenzene (p-chlorotoluene).
Structure of X:
Cl
|
C6H4
|
CH3 (at para position)
(Smiles: ClC1=CC=C(C)C=C1)
Step 2: Benzylic Bromination
Product X (1-chloro-4-methylbenzene) reacts with N-bromosuccinimide (NBS) in carbon tetrachloride (CCl₄). NBS in CCl₄ is a reagent used for selective free-radical halogenation at allylic or benzylic positions.
In 1-chloro-4-methylbenzene, the methyl group attached to the benzene ring has benzylic hydrogens. NBS will replace one of these benzylic hydrogens with a bromine atom.
Therefore, product Y is 1-(bromomethyl)-4-chlorobenzene.
Structure of Y:
Cl
|
C6H4
|
CH2Br (at para position)
(Smiles: ClC1=CC=C(CBr)C=C1)
Step 3: Nucleophilic Substitution
Product Y (1-(bromomethyl)-4-chlorobenzene) reacts with aqueous potassium hydroxide (aq. KOH). Aqueous KOH provides hydroxide ions (OH⁻), which are strong nucleophiles. This reaction is a nucleophilic substitution, where the bromine atom is replaced by a hydroxyl group (-OH).
The -CH₂Br group is a primary benzylic halide, which readily undergoes SN2 reaction with strong nucleophiles like OH⁻. The chlorine atom directly attached to the benzene ring is unreactive under these conditions.
Therefore, product Z is 1-(hydroxymethyl)-4-chlorobenzene, also known as 4-chlorobenzyl alcohol.
Structure of Z:
Cl
|
C6H4
|
CH2OH (at para position)
(Smiles: ClC1=CC=C(CO)C=C1)
Comparing this structure with the given options, option 2 matches the structure of 4-chlorobenzyl alcohol.
The final answer is Option 2.
Explanation of the solution:
-
Chlorobenzene undergoes Friedel-Crafts alkylation with CH₃Cl/AlCl₃. Chlorine is an ortho-para director, and due to steric hindrance, the para-substituted product (1-chloro-4-methylbenzene, X) is major.
-
X undergoes benzylic bromination with NBS/CCl₄. NBS selectively brominates the benzylic hydrogens of the methyl group, forming 1-(bromomethyl)-4-chlorobenzene (Y).
-
Y undergoes nucleophilic substitution with aqueous KOH. The bromine atom of the -CH₂Br group is replaced by a hydroxyl group, yielding 1-(hydroxymethyl)-4-chlorobenzene (4-chlorobenzyl alcohol, Z). The chlorine on the benzene ring remains unaffected.
