Question
Question: $\longrightarrow$ HO-X + H$^+$ $\longrightarrow$...
⟶ HO-X + H+ ⟶
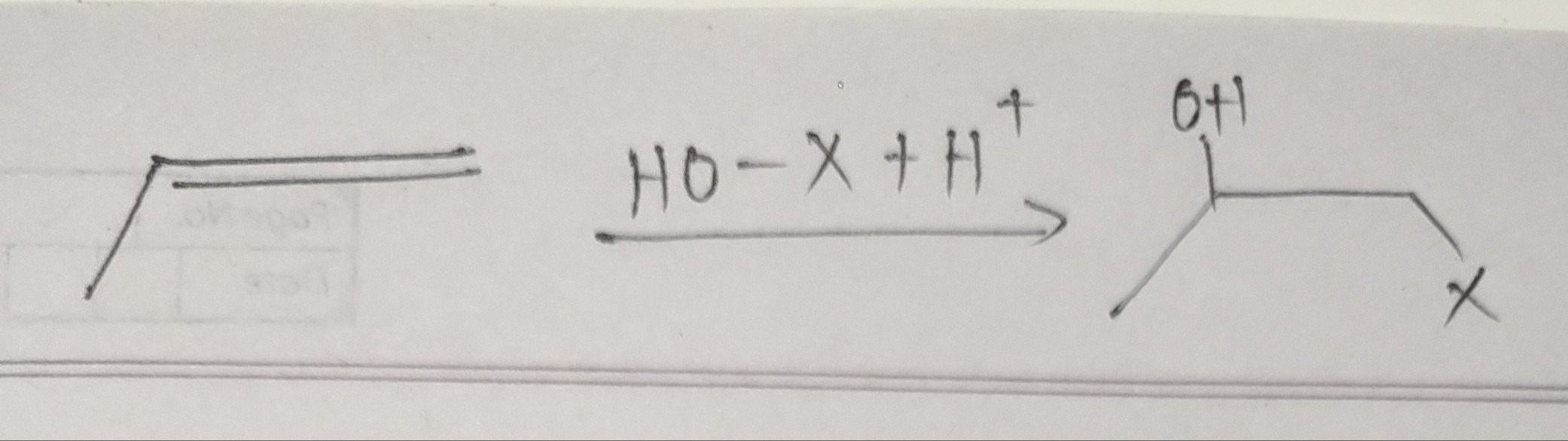
Answer
The reaction shown is the formation of a halohydrin from propene and HO-X. The product is 1-halo-2-propanol.
Explanation
Solution
The reaction is an electrophilic addition of HO-X to propene. The addition follows Markovnikov's rule, where the hydroxyl group (-OH) adds to the more substituted carbon of the double bond, and the halogen atom (-X) adds to the less substituted carbon. This leads to the formation of a halohydrin.
