Question
Question: IUPAC Name ?...
IUPAC Name ?
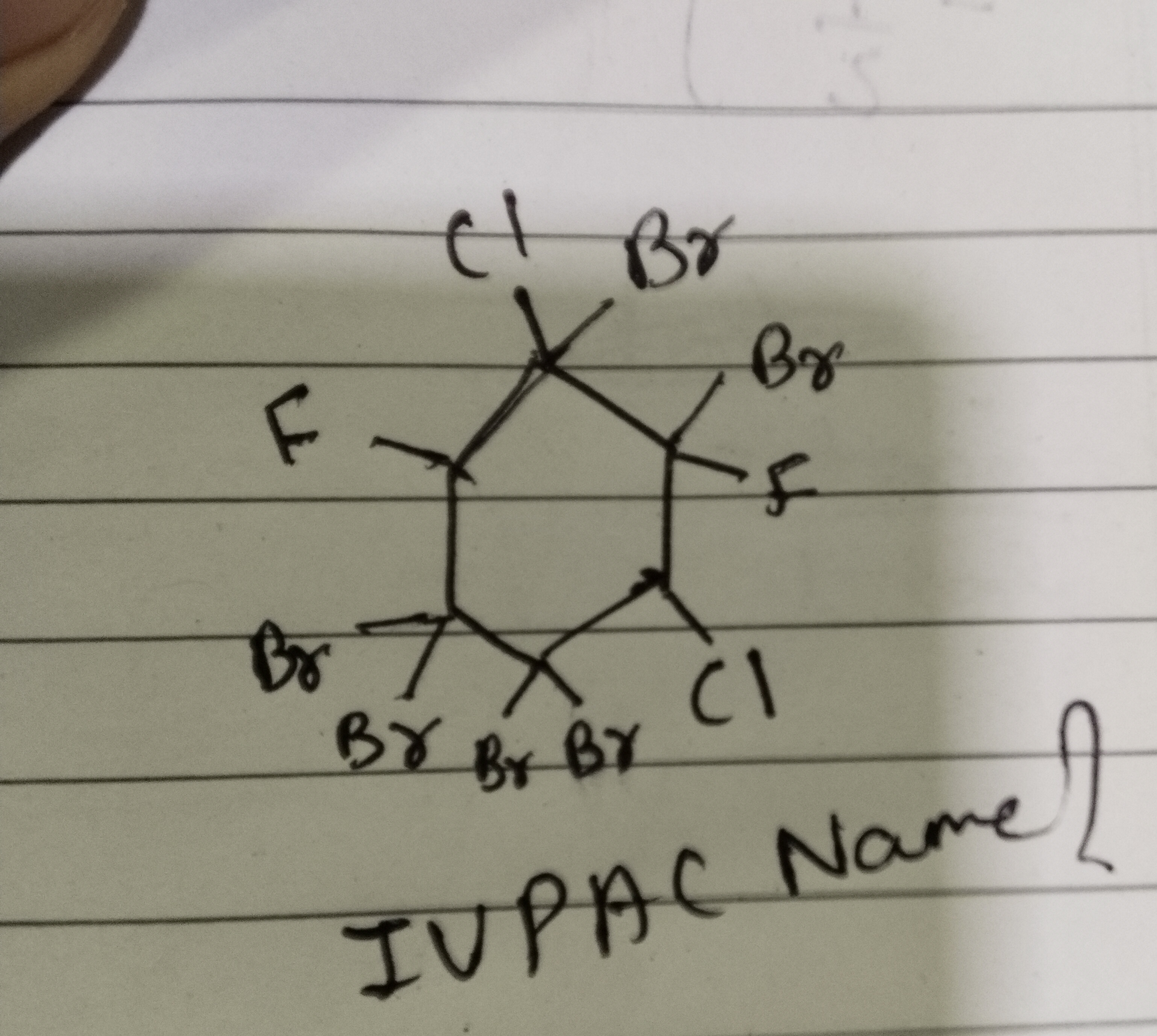
1,1,2,2,3,4,5,6-octabromo-3,6-dichloro-4,5-difluorocyclohexane
Solution
The structure is a cyclohexane ring. Following IUPAC nomenclature rules, we number the ring to give the lowest possible locant set to the substituents, prioritizing alphabetical order. The substituents are bromine (Br), chlorine (Cl), and fluorine (F). Numbering from the bottom-left carbon (C5 in the diagram) clockwise, we get the following assignments: C1 (position 1): 1,1-dibromo C2 (position 2): 2,2-dibromo C3 (position 3): 3-bromo, 3-chloro C4 (position 4): 4-bromo, 4-fluoro C5 (position 5): 5-bromo, 5-fluoro C6 (position 6): 6-bromo, 6-chloro This gives a total of 8 bromines, 2 chlorines, and 2 fluorines. Alphabetical order: Bromo, Chloro, Fluoro. Locants for Bromo: 1,1,2,2,3,4,5,6 Locants for Chloro: 3,6 Locants for Fluoro: 4,5 The IUPAC name is: 1,1,2,2,3,4,5,6-octabromo-3,6-dichloro-4,5-difluorocyclohexane. Note: This structure is chemically impossible as some carbons have more than two substituents. The answer is based on a literal interpretation of the provided, likely erroneous, diagram.
