Question
Question: IUPAC Name...
IUPAC Name
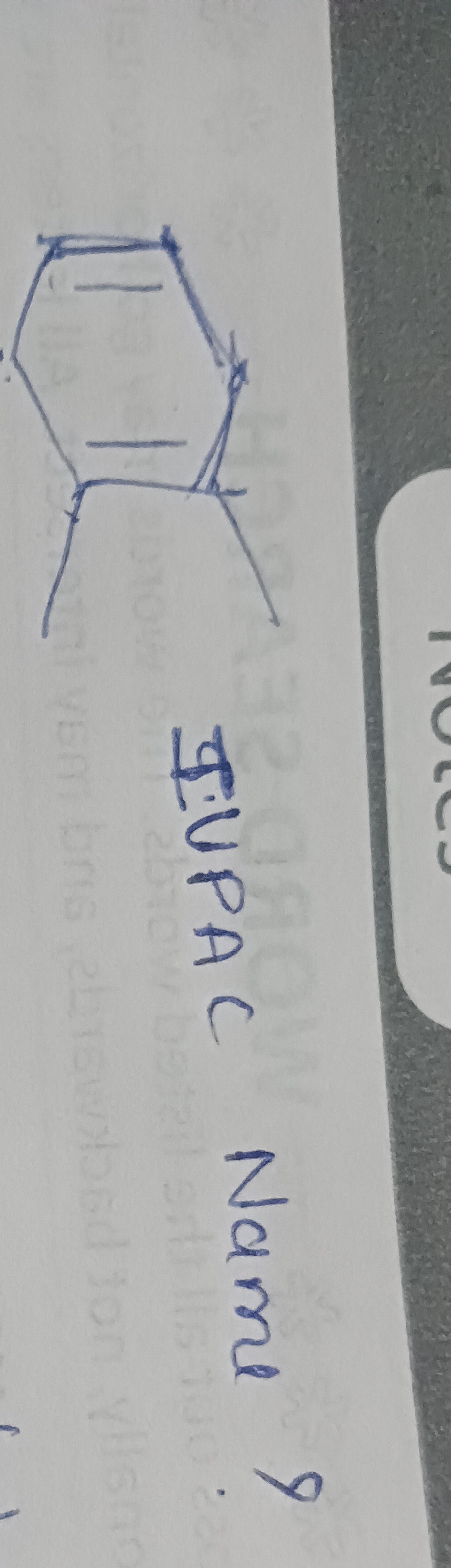
Answer
1,4-dimethyl-1,3-cyclohexadiene
Explanation
Solution
The given structure is a cyclohexadiene with two methyl substituents. According to IUPAC rules, the ring carbons are numbered to give the double bonds the lowest possible locants. If there's a choice, substituents get the lowest locants.
-
Identify the parent diene: It's a six-membered ring with two double bonds, so cyclohexadiene.
-
Numbering for lowest double bond locants:
- If double bonds are at 1,4 (as visually suggested by horizontal lines), and methyls are at 2,5, the name is 2,5-dimethyl-1,4-cyclohexadiene. Double bond locants: (1,4).
- If we number to place double bonds at 1,3, and methyls at 1,4, the name is 1,4-dimethyl-1,3-cyclohexadiene. Double bond locants: (1,3).
-
Prioritize lowest double bond locants: The set (1,3) is lower than (1,4). Therefore, 1,4-dimethyl-1,3-cyclohexadiene is the correct naming.
