Question
Question: The reaction of $\alpha$-bromophenylacetone with $\text{NaBH}_4$ in $\text{CH}_3\text{OH}$ followed...
The reaction of α-bromophenylacetone with NaBH4 in CH3OH followed by treatment with ethylmagnesium bromide and then H3O+ yields which of the following products?
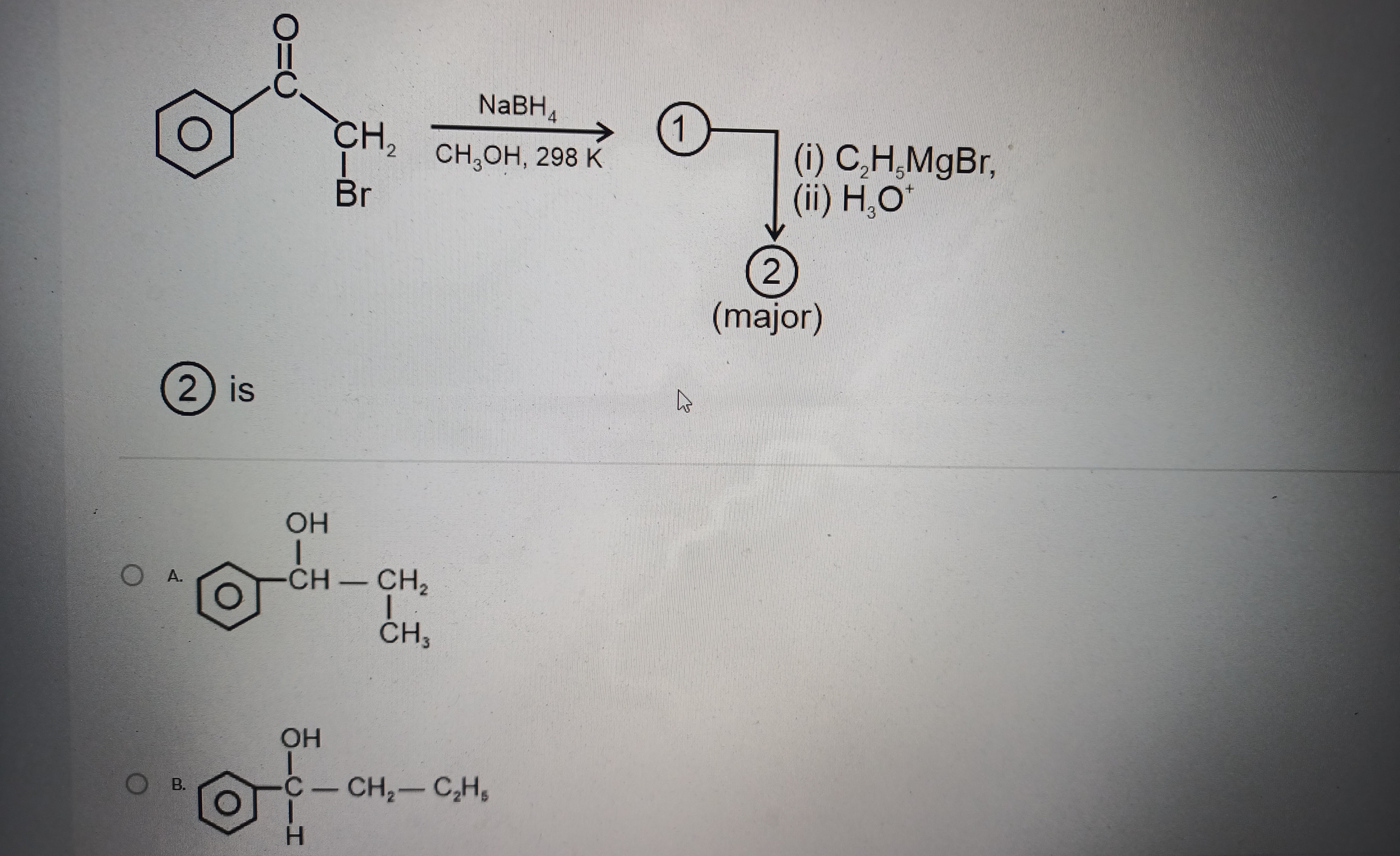
A
OH
|
Ph - CH - CH2 - CH3
B
OH
|
Ph - CH - CH2 - CH2 - CH3
C
OH
|
Ph - CH - CH2 - CH2 - CH2 - CH3
D
OH
|
Ph - CH - CH2 - CH2 - CH2 - CH2 - CH3
Answer
OH
|
Ph - CH - CH2 - CH2 - CH3
Explanation
Solution
- Reduction of ketone: α-bromophenylacetone is reduced by NaBH4 to 2-bromo-1-phenylethanol. PhCOCH2BrNaBH4/CH3OHPhCH(OH)CH2Br
- Reaction with Grignard reagent: The Grignard reagent (C2H5MgBr) reacts with the acidic proton of the alcohol and then displaces the bromide via an SN2 reaction. PhCH(OH)CH2Br1. C2H5MgBr (excess)2. H3O+PhCH(OH)CH2C2H5 The product is 1-phenylpentan-1-ol.
