Question
Question: If three reactions: A → Products Rate = K1[A]^X B → Products Rate = K2[B]^Y C → Products Rate = K3[C...
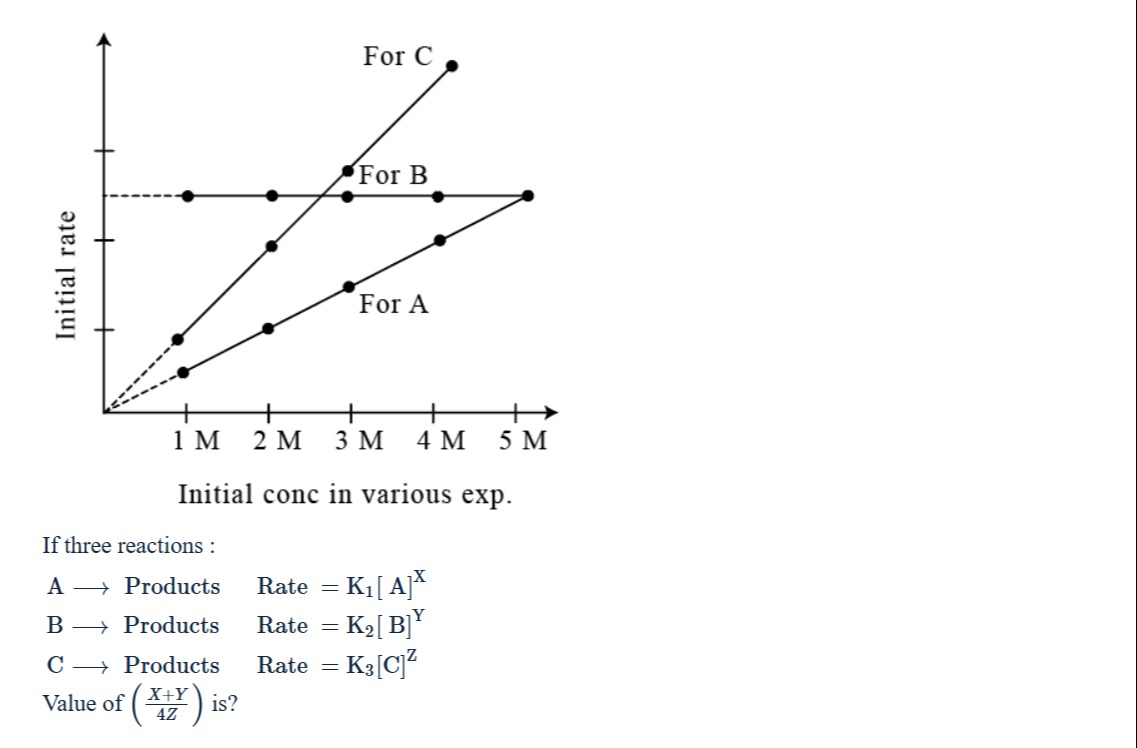
If three reactions: A → Products Rate = K1[A]^X B → Products Rate = K2[B]^Y C → Products Rate = K3[C]^Z Value of (4ZX+Y) is?
0.25
Solution
The given reactions are:
A → Products, Rate = K1[A]X B → Products, Rate = K2[B]Y C → Products, Rate = K3[C]Z
We need to determine the values of X, Y, and Z from the given graph, which plots the initial rate versus the initial concentration.
For reaction A: The graph for A is a straight line passing through the origin. This indicates that the initial rate is directly proportional to the initial concentration of A. The rate law is Rate = K1[A]X. If the rate is directly proportional to [A], then X=1.
Let's verify this from the graph. Let the initial rate at [A] = 1 M be R0. From the graph, it appears that the rate at [A] = 2 M is 2R0, at [A] = 3 M is 3R0, and at [A] = 4 M is 4R0. Substituting these values into the rate law: At [A] = 1 M, R0=K1(1)X At [A] = 2 M, 2R0=K1(2)X Dividing the second equation by the first: R02R0=K1(1)XK1(2)X, which simplifies to 2=2X. This gives X=1. Thus, the order of reaction A with respect to A is X=1.
For reaction B: The graph for B shows that the initial rate is constant for initial concentrations of B from 1 M to 4 M. The rate law is Rate = K2[B]Y. If the rate is independent of the concentration of B, then Y=0. From the graph, the rate for B is constant at a certain value, which appears to be the same as the rate for A at [A] = 4 M, which is 4R0. So, Rate = 4R0 for [B] ≥ 1 M. Substituting into the rate law: 4R0=K2[B]Y. Since the rate is constant for different values of [B], the exponent Y must be 0. 4R0=K2[B]0=K2. Thus, the order of reaction B with respect to B is Y=0.
For reaction C: The graph for C is a straight line passing through the origin. This indicates that the initial rate is directly proportional to the initial concentration of C raised to some power Z. Since it's a straight line through the origin on a linear plot, the power Z must be 1. The rate law is Rate = K3[C]Z. Let's verify this from the graph. The line for C passes through the origin (0, 0). It also passes through the point where [C] = 2 M and the rate is the same as the rate for B, which is 4R0. So, at [C] = 2 M, Rate = 4R0. Substituting into the rate law: 4R0=K3(2)Z. Let's look at another point on the line for C. At [C] = 4 M, the rate appears to be 8R0 (twice the rate at [C] = 2 M, consistent with a linear relationship through the origin). So, at [C] = 4 M, Rate = 8R0. Substituting into the rate law: 8R0=K3(4)Z. We have two equations:
- 4R0=K32Z
- 8R0=K34Z Dividing equation 2 by equation 1: 4R08R0=K32ZK34Z, which simplifies to 2=2Z4Z=2Z(22)Z=2Z22Z=22Z−Z=2Z. So, 2=2Z, which gives Z=1. Thus, the order of reaction C with respect to C is Z=1.
We have found the values of X, Y, and Z: X=1 Y=0 Z=1
We need to find the value of (4ZX+Y). Substitute the values of X, Y, and Z into the expression: (4ZX+Y)=(4×11+0)=(41)=0.25.
The final answer is 0.25.
Explanation of the solution:
- Analyze the graph for reaction A to determine the order X. The linear relationship through the origin indicates a first-order reaction, so X=1.
- Analyze the graph for reaction B to determine the order Y. The constant rate for varying concentrations indicates a zero-order reaction, so Y=0.
- Analyze the graph for reaction C to determine the order Z. The linear relationship through the origin indicates a first-order reaction, so Z=1.
- Substitute the determined values of X, Y, and Z into the expression (4ZX+Y) and calculate the result.
