Question
Question: Identify major product (P) in above reaction....
Identify major product (P) in above reaction.
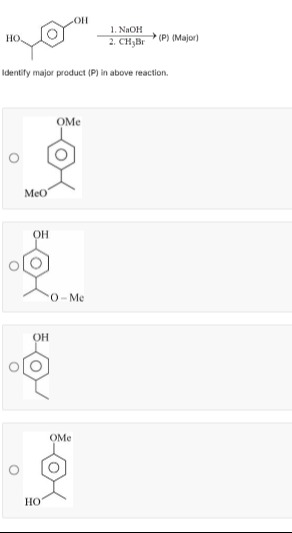
Shows both -OH groups methylated.
Shows the alcoholic -OH methylated and the phenolic -OH unchanged.
Shows the alcoholic -OH removed and the phenolic -OH unchanged.
Shows the phenolic -OH methylated to -OCH₃ and the alcoholic -OH unchanged.
Option 4
Solution
The reaction involves two steps:
-
Reaction with NaOH: The starting material, 4-(1-hydroxyethyl)phenol, contains two types of hydroxyl groups: a phenolic -OH (directly attached to the benzene ring) and a secondary alcoholic -OH (on the side chain). Phenolic hydroxyl groups are significantly more acidic (pKa ≈ 10) than aliphatic alcoholic hydroxyl groups (pKa ≈ 16-18) due to the resonance stabilization of the phenoxide ion. Sodium hydroxide (NaOH) is a strong base, but it is not strong enough to deprotonate an alcohol to a significant extent. Therefore, NaOH will selectively deprotonate the more acidic phenolic -OH group, forming a phenoxide ion. The alcoholic -OH group remains unchanged.
\text{HO-}C_6H_4\text{-CH(OH)-CH}_3 \xrightarrow{NaOH} \text{Na}^+\text{ }^-\text{O-}C_6H_4\text{-CH(OH)-CH}_3 + H_2O -
Reaction with CH₃Br: Methyl bromide (CH₃Br) is a primary alkyl halide and an excellent substrate for SN2 reactions. The phenoxide ion formed in the first step is a strong nucleophile. It will react with CH₃Br via a Williamson ether synthesis, displacing the bromide ion and forming a methyl ether. The alcoholic -OH group, which was not deprotonated, will not react with CH₃Br under these conditions.
\text{Na}^+\text{ }^-\text{O-}C_6H_4\text{-CH(OH)-CH}_3 + CH_3Br \xrightarrow{SN2} \text{CH}_3\text{O-}C_6H_4\text{-CH(OH)-CH}_3 + NaBr
Therefore, the major product (P) will have the phenolic hydroxyl group methylated to a methoxy group (-OCH₃), while the secondary alcoholic hydroxyl group remains intact as -OH.
The structure of the major product (P) is 4-(1-hydroxyethyl)anisole.
Explanation of the solution:
- NaOH deprotonates the more acidic phenolic -OH, forming a phenoxide ion. The alcoholic -OH remains unchanged.
- The phenoxide ion acts as a nucleophile and undergoes SN2 reaction with CH₃Br (Williamson ether synthesis) to form a methyl ether.
- The resulting product has a methoxy group on the benzene ring and an unchanged hydroxyl group on the side chain.
