Question
Question: The reaction shown is the electrophilic addition of HCl to an alkene. The alkene has an exocyclic do...
The reaction shown is the electrophilic addition of HCl to an alkene. The alkene has an exocyclic double bond. The double bond is between a quaternary carbon in the ring and an exocyclic carbon which is bonded to a methyl group and a hydrogen atom. According to Markovnikov's rule, the proton (H+) from HCl will add to the carbon atom of the double bond that has more hydrogen atoms. In this case, the exocyclic carbon has one hydrogen atom, while the quaternary ring carbon has none. Therefore, the proton adds to the exocyclic carbon, forming a carbocation on the quaternary ring carbon. This carbocation is relatively stable due to its tertiary/quaternary nature. The chloride ion (Cl-) then attacks the carbocation, leading to the formation of the final product where the chlorine atom is attached to the quaternary ring carbon and a hydrogen atom is attached to the exocyclic carbon.
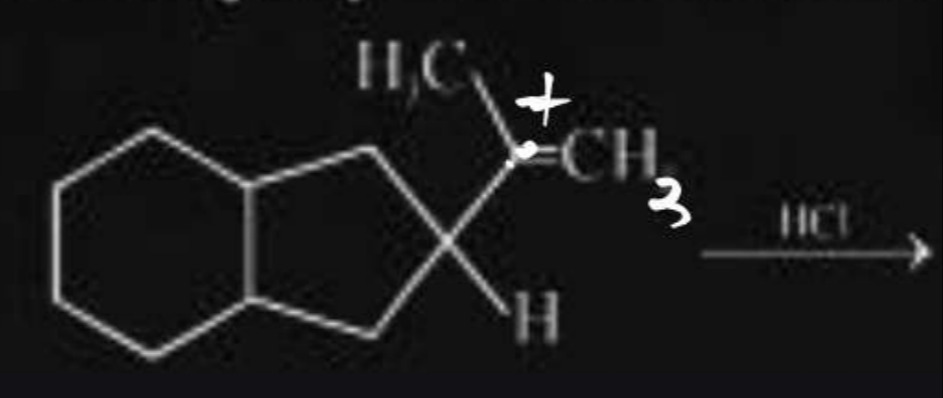
The product is formed by the addition of H to the exocyclic carbon and Cl to the quaternary ring carbon.
Solution
The reaction is an electrophilic addition of HCl to an alkene. According to Markovnikov's rule, the proton (H+) adds to the carbon of the double bond with more hydrogen atoms. In this case, the exocyclic carbon has one hydrogen and the ring carbon has none. Thus, H+ adds to the exocyclic carbon, forming a carbocation on the quaternary ring carbon. The chloride ion (Cl-) then attacks the carbocation, resulting in the chlorine atom attached to the quaternary ring carbon.
