Question
Question: Half-life is independent of conc. of A. After 10 minutes volume N₂ gas is 10 L and after complete re...
Half-life is independent of conc. of A. After 10 minutes volume N₂ gas is 10 L and after complete reaction 50 L. Hence rate constant in min⁻¹:
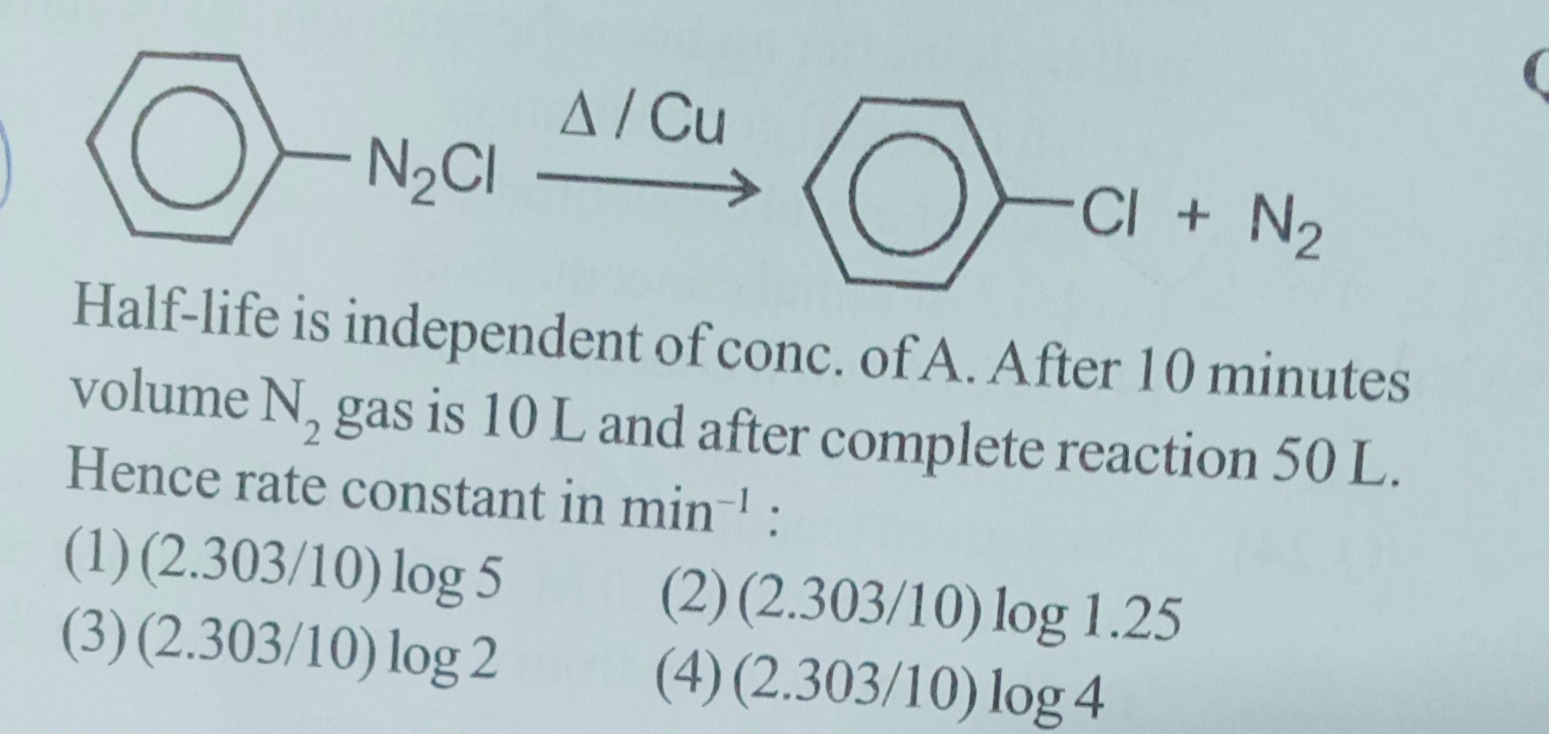
(2.303/10) log 5
(2.303/10) log 1.25
(2.303/10) log 2
(2.303/10) log 4
(2.303/10) log 1.25
Solution
The half-life of the reaction is independent of the concentration of reactant A, which means the reaction is a first-order reaction with respect to A. The reaction is the decomposition of benzene diazonium chloride (C6H5N2Cl) to chlorobenzene (C6H5Cl) and nitrogen gas (N2). The reaction is:
C6H5N2Cl→C6H5Cl+N2
For a first-order reaction, the integrated rate law is given by:
k=t2.303log[A]t[A]0
where k is the rate constant, t is the time, [A]0 is the initial concentration of reactant A, and [A]t is the concentration of reactant A at time t.
In this reaction, nitrogen gas (N2) is produced. The volume of N2 gas produced is proportional to the amount of reactant A that has decomposed. Let Vt be the volume of N2 gas produced at time t, and V∞ be the volume of N2 gas produced after the complete reaction.
The initial amount of reactant A is proportional to the total amount of N2 that can be produced, which is proportional to V∞. So, [A]0∝V∞.
The amount of reactant A that has reacted at time t is proportional to the volume of N2 produced at time t, which is Vt.
The amount of reactant A remaining at time t, [A]t, is proportional to the initial amount minus the amount reacted. Thus, [A]t∝V∞−Vt.
Substituting these proportionalities into the first-order rate equation:
k=t2.303log[A]t[A]0=t2.303logV∞−VtV∞
We are given:
Time t=10 minutes.
Volume of N2 gas at t=10 minutes is V10=10 L.
Volume of N2 gas after complete reaction is V∞=50 L.
Substitute these values into the rate constant equation:
k=102.303log50−1050=102.303log4050=102.303log45=102.303log1.25
