Question
Question: $\frac{Br_2}{dark} \longrightarrow P$ ...
darkBr2⟶P
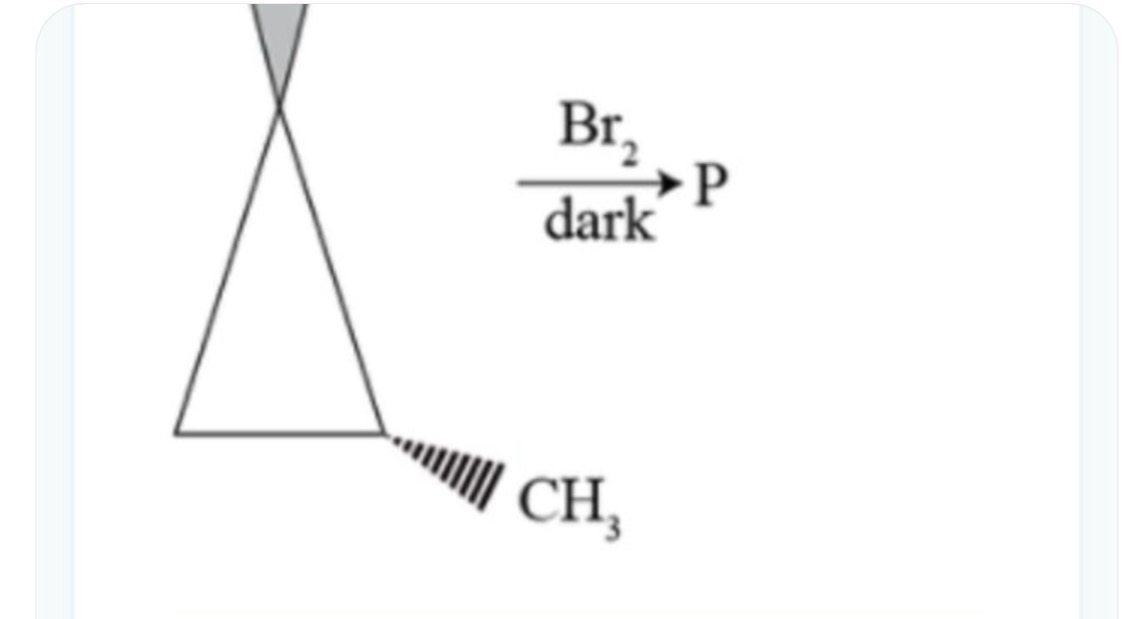
Product P is 1,3–dibromo–2–methylpropane.
Solution
Solution:
The reaction is an electrophilic “opening” of a cyclopropane. Under Br₂ in the dark the electrophile (Br⁺) attacks the strained cyclopropane (which, though “saturated,” has bent bonds that are unusually reactive). When the electrophile adds (via formation of a cyclic bromonium‐ion intermediate) the ring opens. In our substrate the cyclopropane has a CH₃ substituent at one carbon and a Br at another. The bromonium–ion is formed over the more electron‐rich bond so that when Br⁻ attacks the intermediate (anti–attack common for such additions) the ring opens to give an open–chain dibromide.
Careful analysis of the regioselectivity (the CH₃ group helps stabilize any developing positive character) shows that the resulting product is
1,3–dibromo–2–methylpropane.Thus, product \textbf{P} is 1,3–dibromo–2–methylpropane.
Minimal Explanation (Core Steps):
-
Br₂ adds electrophilically to the cyclopropane (which is reactive due to ring strain) forming a bromonium ion.
-
The ring opens via backside attack by Br⁻.
-
Regioselectivity is governed by stabilization from the CH₃ group → product: 1,3–dibromo–2–methylpropane.
