Question
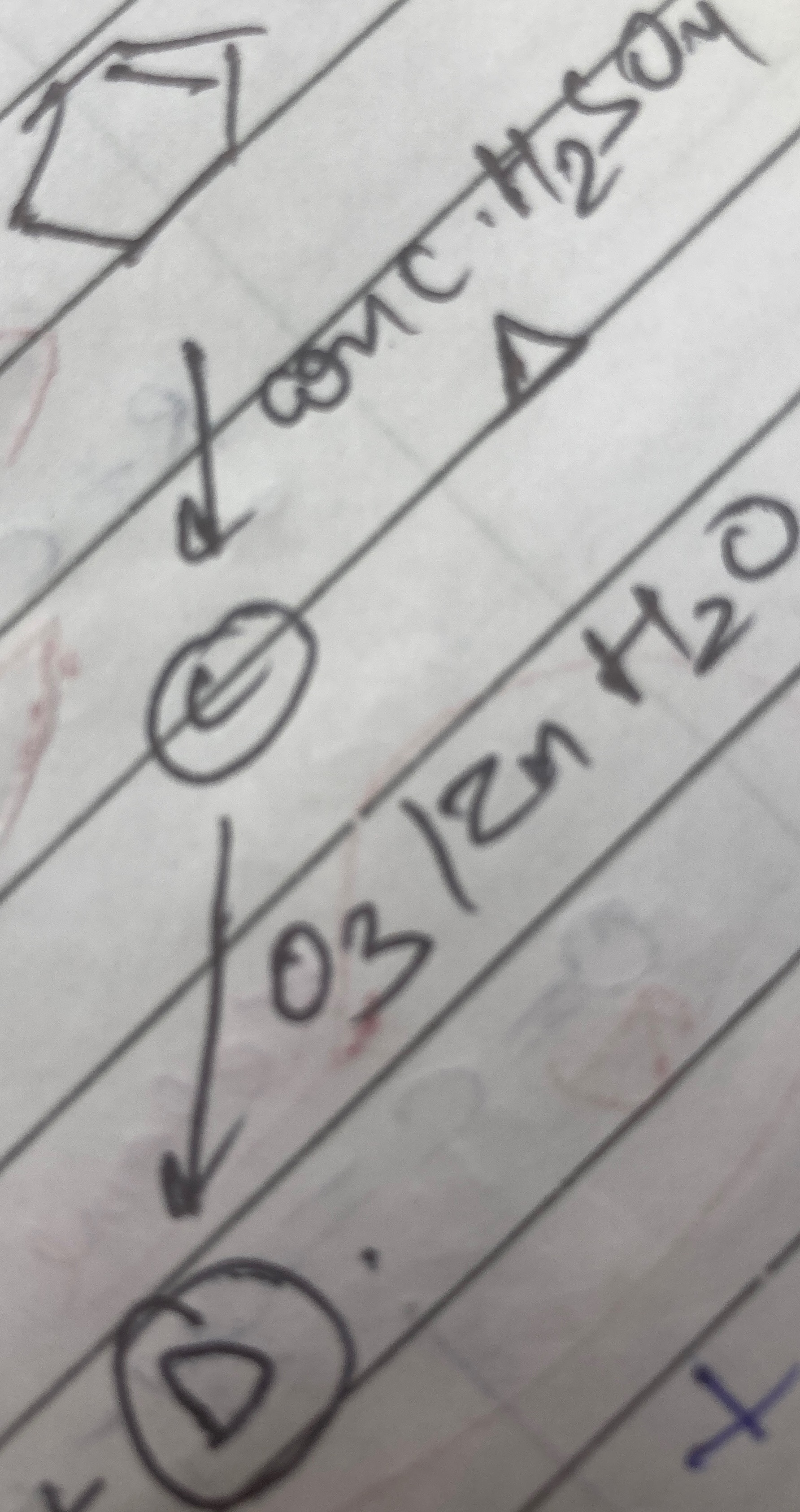
Question: $\downarrow$ conc. $H_2SO_4$, $\Delta$ (C) $\downarrow$ $O_3/Zn$ $H_2O$ (D)...
↓ conc. H2SO4, Δ
(C)
↓ O3/Zn H2O
(D)
1,2,3,4-hexanetetraldehyde
Solution
The initial molecule is cyclohex-1-en-3-ol.
Reaction with conc. H2SO4 and Δ causes dehydration. The -OH group is removed from C3, and a hydrogen atom is removed from an adjacent carbon. The adjacent carbons are C2 and C4. Removing a hydrogen from C2 (which is part of the double bond) is less favorable than removing a hydrogen from C4. Removing a hydrogen from C4 forms a double bond between C3 and C4. This leads to the formation of cyclohex-1,3-diene (C).
Cyclohex-1,3-diene has conjugated double bonds.
Next, cyclohex-1,3-diene undergoes ozonolysis with reductive workup (O3/Zn H2O). Ozonolysis cleaves the double bonds and converts the carbon atoms of the double bond into carbonyl groups. Since the workup is reductive (with Zn/H2O), aldehydes and ketones are formed.
The double bonds in cyclohex-1,3-diene are between C1 and C2, and between C3 and C4.
Cleavage of C1=C2 gives C1=O and C2=O. Cleavage of C3=C4 gives C3=O and C4=O.
The single bonds are C2-C3, C4-C5, C5-C6, C6-C1.
Let's connect the fragments:
C1=O is connected to C6. C2=O is connected to C3. C3=O is connected to C2 and C4. C4=O is connected to C3 and C5. C5 is connected to C4 and C6. C6 is connected to C5 and C1.
The linear chain formed is O=C1-C6-C5-C4=O-C3=O-C2=O. This is a hexanal with aldehyde groups at positions 1, 2, 3, 4. Let's write the structure: O=CH-CH=O-CH=O-CH=O-CH2-CH2-. This is 1,2,3,4-hexanetetraldehyde.
