Question
Question: Correct statements for above reaction sequence:...
Correct statements for above reaction sequence:
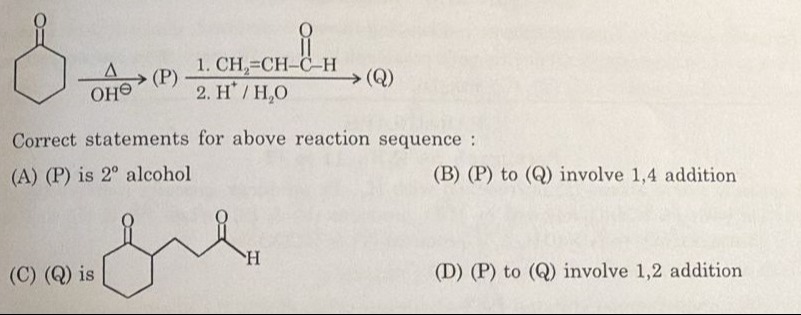
(P) is 2º alcohol
(P) to (Q) involve 1,4 addition
(Q) is
(P) to (Q) involve 1,2 addition
B, C
Solution
The reaction sequence starts with cyclohexanone reacting under basic conditions with heat, followed by reaction with propenal (CH2=CH-CHO) and then acidification.
Step 1: Cyclohexanone Δ,OH− (P)
Under basic conditions, cyclohexanone undergoes enolization to form the enolate. Heating promotes the formation of the enolate and subsequent reactions. Given the next step, it is most likely that (P) is the enolate of cyclohexanone, which acts as a nucleophile in the subsequent reaction.
Step 2: (P) + CH2=CH-CHO 2.H+/H2O (Q)
The reaction of an enolate with an α,β-unsaturated carbonyl compound is a Michael addition, which is a type of 1,4 addition. The enolate of cyclohexanone acts as a nucleophile and attacks the β-carbon of propenal. This forms a new carbon-carbon bond and an enolate intermediate. Subsequent protonation of the enolate oxygen and tautomerization leads to the final product (Q).
Let's draw the structures and reaction:
Cyclohexanone enolate:
O⁻
|
C=C - C - C - C - C
|
(ring, double bond exocyclic or endocyclic)
Let's use the enolate with the negative charge on the carbon adjacent to the carbonyl:
O
||
/ \ / \
| C⁻ - C
| | |
\ / \ / C=O
\ / / \
C----C
Propenal: CH2=CH-CHO
Michael addition (1,4 addition):
The nucleophilic carbon of the enolate attacks the β-carbon (CH2) of propenal.
O
||
/ \ / \
| C - C -- CH₂ - CH = C - O⁻
| | |
\ / \ / C=O
\ / / \
C----C
(intermediate enolate)
Acid workup (H+/H2O):
Protonation of the intermediate enolate and tautomerization to the keto form gives (Q).
O
||
/ \ / \
| C - C -- CH₂ - CH₂ - C = O
| | | |
\ / \ / C=O H
\ / / \
C----C
So, (Q) is 2-(3-oxopropyl)cyclohexanone.
Now let's evaluate the statements:
(A) (P) is 2º alcohol. If (P) is the enolate, it is not an alcohol. If (P) were the Aldol addition product of cyclohexanone with itself, it would be a 3º alcohol. So, (A) is incorrect.
(B) (P) to (Q) involve 1,4 addition. The reaction of the enolate (P) with propenal is a Michael addition, which is a 1,4 addition to the α,β-unsaturated system. This statement is correct.
(C) (Q) is
This structure is 2-(3-oxopropyl)cyclohexanone, which is the product we derived. This statement is correct.
(D) (P) to (Q) involve 1,2 addition. 1,2 addition would be attack on the carbonyl carbon of propenal. Michael addition is 1,4 addition. While 1,2 addition is possible, 1,4 addition is the favored pathway for soft nucleophiles like enolates with α,β-unsaturated carbonyl compounds. The product structure confirms 1,4 addition. This statement is incorrect.
Therefore, the correct statements are (B) and (C).
