Question
Question: Cl₂/hv → ? Total number of product in above reaction will be...
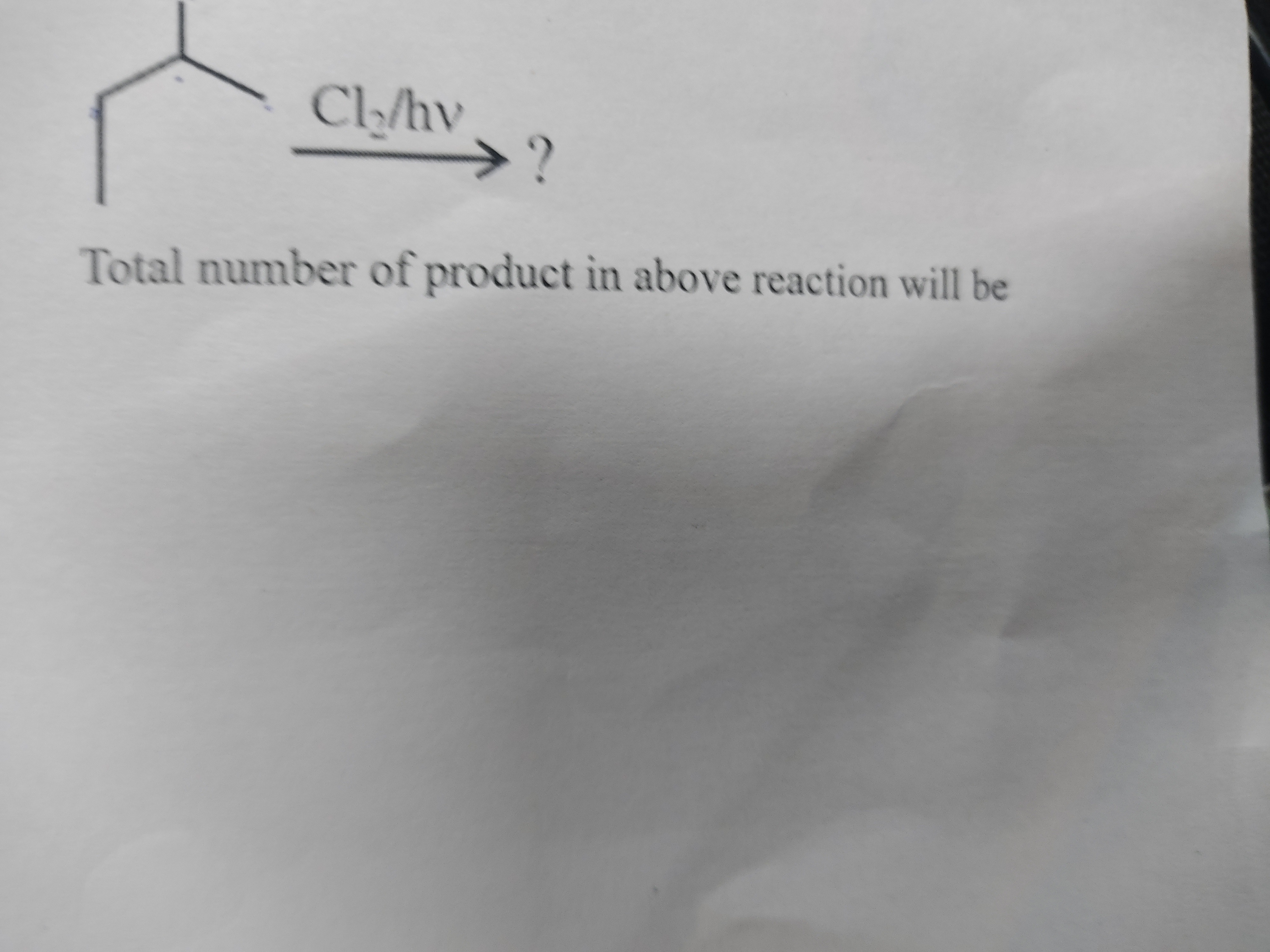
Cl₂/hv → ?
Total number of product in above reaction will be
Answer
5
Explanation
Solution
The reaction is a free radical chlorination of 2-methylpentane using Cl₂ and hv (light). This process involves the substitution of hydrogen atoms by chlorine atoms. We need to identify the number of distinct monochlorinated products.
The structure of 2-methylpentane is:
There are five distinct types of hydrogen atoms based on their chemical environment:
- Hydrogens on C1 and C6: These are primary hydrogens on equivalent methyl groups. Substitution yields 1-chloro-2-methylpentane.
- Hydrogen on C2: This is a tertiary hydrogen. Substitution yields 2-chloro-2-methylpentane.
- Hydrogens on C3: These are secondary hydrogens. Substitution yields 3-chloro-2-methylpentane.
- Hydrogens on C4: These are secondary hydrogens. Substitution yields 4-chloro-2-methylpentane.
- Hydrogens on C5: These are primary hydrogens. Substitution yields 5-chloro-2-methylpentane.
Since there are five distinct types of hydrogen atoms, there will be five different monochlorinated products.
