Question
Question: HOOC- HOOC COOH COOH Calculate total moles of $CO_2$ evolved per mol substrate ...
HOOC-
HOOC COOH
COOH
Calculate total moles of CO2 evolved per mol substrate
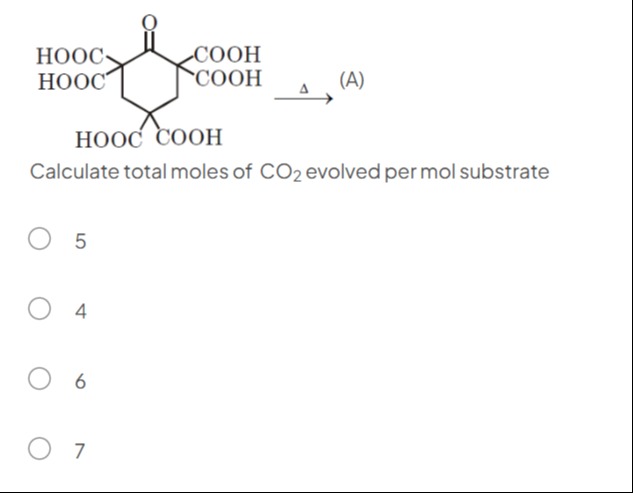
A
5
B
4
C
6
D
7
Answer
6
Explanation
Solution
Solution
The starting molecule is a cyclic compound having six substituents on the ring – five are directly –COOH groups and one is in the form HOOC–. Under thermal (and catalytic) conditions the decarboxylation reaction takes place. In decarboxylation, each carboxylic acid group (–COOH) is eliminated as CO₂. Here all six –COOH type groups are lost. Thus, per mole of the substrate, 6 moles of CO₂ are evolved.
Minimal Explanation
-
The substrate carries six carboxyl groups (five –COOH and one HOOC–).
-
Under decarboxylation each –COOH group gives one CO₂.
-
Total CO₂ = 6 moles per mole of substrate.
