Question
Question: (i) AlCl3 A (ii) Zn(Hg)/HCl (iii) H3PO4 A (i) Zn-Hg/HCl B H2 (excess) C (ii) Se/Δ Pt/Δ...
(i)
AlCl3 A (ii) Zn(Hg)/HCl (iii) H3PO4
A (i) Zn-Hg/HCl B H2 (excess) C (ii) Se/Δ Pt/Δ
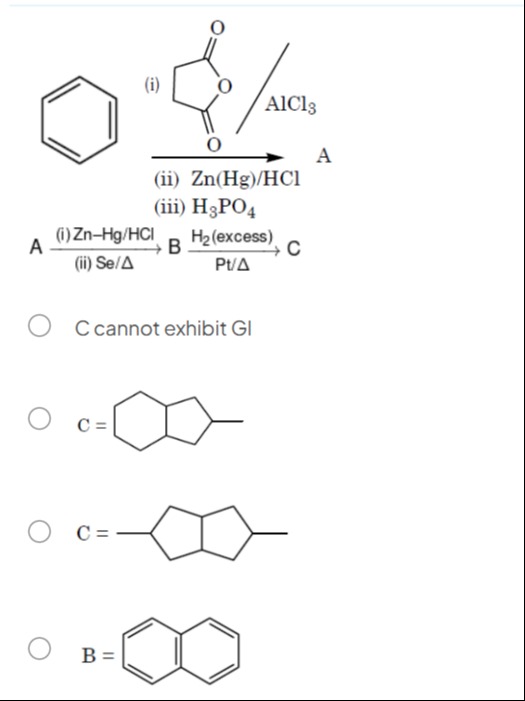
C cannot exhibit Gl
C =
C =
B =
Compound B is 2–methylindene and Compound C is 2–methylindane
Solution
We shall show briefly how one may “read‐across” the reaction sequence.
-
The anhydride used is succinic anhydride (a five‐membered ring with two C=O groups with the “bridge” –OC(O)– present). Under Friedel–Crafts conditions (AlCl₃) benzene is acylated. In the usual “intramolecular acylation” the initially formed acylium ion (from succinic anhydride) undergoes cyclisation to give a cyclic ketone; that is, one obtains 1‐indanone (which has a benzene ring fused to a five‐membered ring containing a CO group).
-
Treatment of 1‐indanone with Zn(Hg)/HCl (Clemmensen reduction) reduces the carbonyl group to –CH₂– so that one obtains indane (a benzene ring fused to a saturated five‐membered ring). Often, using a substituted anhydride (for example, methylsuccinic anhydride) a methyl group is introduced on the methylene part so that the product is 2‐methylindane.
-
When this “alkyl–indane” is treated with Se/Δ a dehydrogenation takes place and the five‐membered ring is “aromatized” to give the corresponding indene (here, 2‐methylindene). Finally, hydrogenation (H₂, Pt/Δ) converts the double bond back giving the saturated indane. (Note that the fully saturated indane cannot “exhibit” the special aromatic “Glauberman–like” behaviour – i.e. it does not have a 4n+2 π–system in both rings.)
Thus one may assign:
- Compound B is the unsaturated (aromatic) indene (namely 2–methylindene).
- Compound C (the hydrogenated product of B) is the indane structure. Among the given drawings the structure that shows a benzene ring fused to a five–membered ring with a methyl substituent on the five–membered ring is the correct one.
Core (minimal) explanation:
-
Benzene + methylsuccinic anhydride (which is a cyclic anhydride with a –CH₃) in the presence of AlCl₃ gives (via intramolecular acylation) 2–methyl–1–indanone.
-
Reduction (Zn–Hg/HCl) converts the C=O to –CH₂– giving 2–methylindane.
-
Dehydrogenation with Se/Δ gives 2–methylindene (B) and subsequent hydrogenation (H₂, Pt/Δ) returns 2–methylindane (C). Since the saturated indane (C) has no conjugated π–system in the five–membered ring it cannot exhibit the aromatic (4n+2) “Glauberman–like” behaviour.
