Question
Question: Arrange the (C-H) bonds x, y and z in decreasing order of their bond dissociation energies in homoly...
Arrange the (C-H) bonds x, y and z in decreasing order of their bond dissociation energies in homolysis.
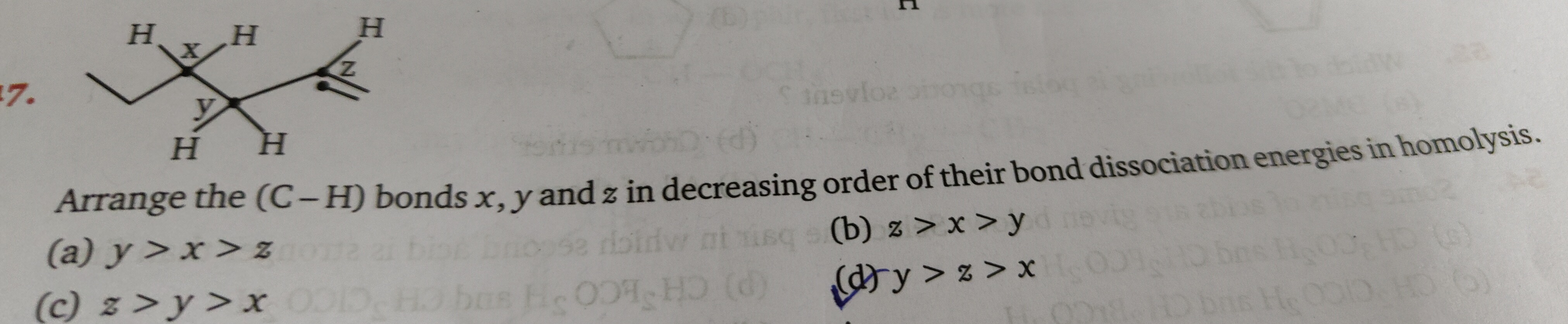
y > x > z
z > x > y
z > y > x
y > z > x
z > x > y
Solution
Bond dissociation energy (BDE) of a C-H bond is inversely related to the stability of the radical formed upon homolytic cleavage. The order of radical stability is generally: tertiary > secondary > primary > vinylic. This is because tertiary radicals are stabilized by hyperconjugation from three alkyl groups, secondary radicals by two, primary by one, and vinylic radicals are destabilized due to the higher electronegativity of the sp2 hybridized carbon atom.
In the given figure:
- Bond x is a C-H bond on a secondary carbon atom (bonded to two other carbon atoms and one hydrogen).
- Bond y is a C-H bond on a tertiary carbon atom (bonded to three other carbon atoms and one hydrogen).
- Bond z is a C-H bond on a vinylic carbon atom (part of a carbon-carbon double bond).
Therefore, the stability of the radicals formed is: radical from y (tertiary) > radical from x (secondary) > radical from z (vinylic). Consequently, the bond dissociation energies are in the reverse order of radical stability: BDE(z) > BDE(x) > BDE(y).
