Question
Question: ⅰ) Armiydrous AlCl3 ⅰ ⅱ) R - Mg x iii) H+....
ⅰ) Armiydrous AlCl3 ⅰ ⅱ) R - Mg x iii) H+.
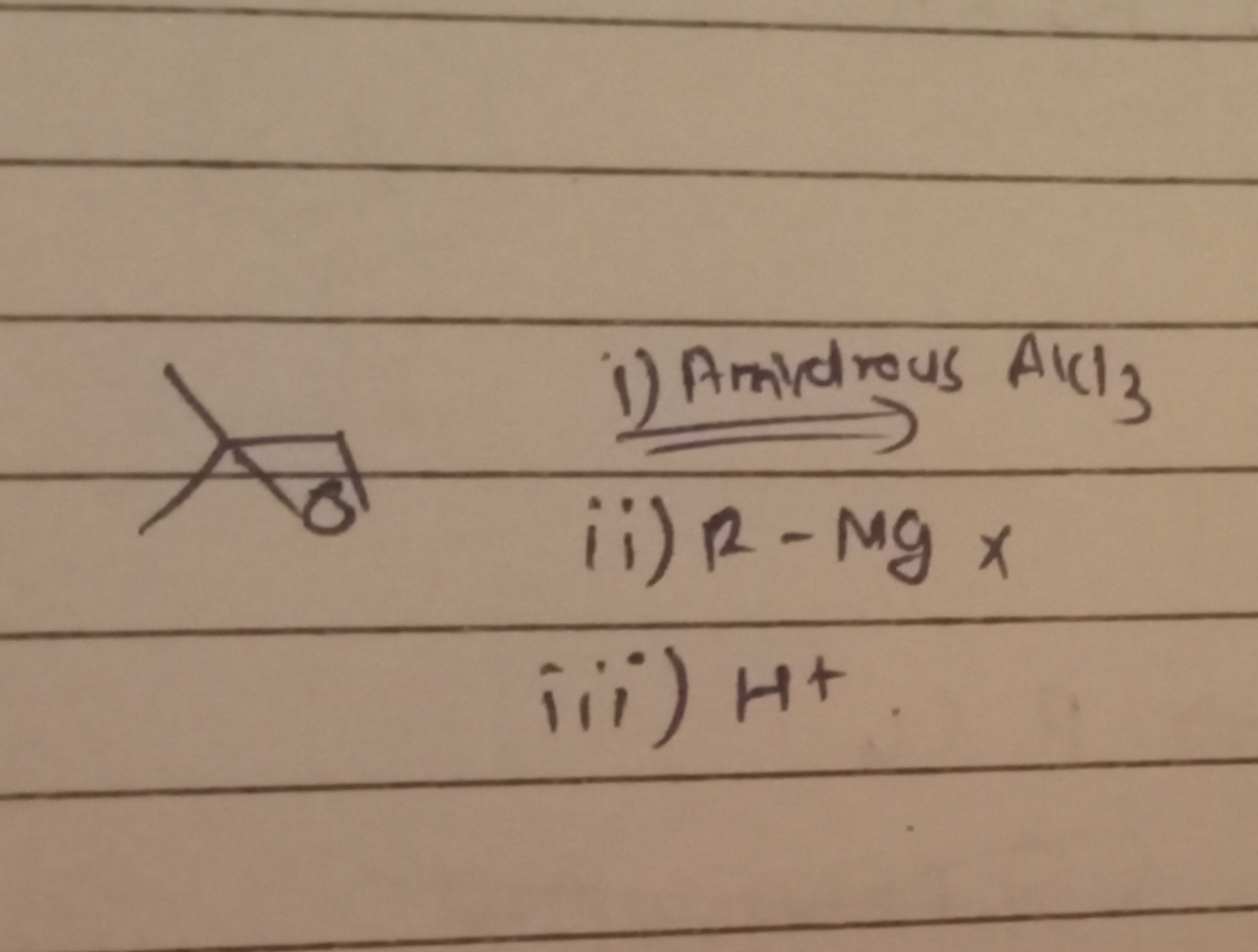
The product of the reaction is R-C(CH3)2-CH2-OH.
Solution
The given reaction involves the ring-opening of an epoxide (2,2-dimethyloxirane) with a Grignard reagent (R-MgX) in the presence of a Lewis acid (anhydrous AlCl3), followed by acidic workup.
1. Identification of the Starting Material: The starting material is a three-membered cyclic ether with two methyl groups attached to one of the ring carbons. This compound is 2,2-dimethyloxirane (also known as 2,2-dimethyloxacyclopropane).
Chemical structure (SMILES): CC1(C)OC1
2. Reaction Mechanism:
-
Step 1: Activation of Epoxide by Lewis Acid (Anhydrous AlCl3) Anhydrous AlCl3 is a Lewis acid. It coordinates with the lone pair of electrons on the oxygen atom of the epoxide. This coordination makes the carbon-oxygen bonds weaker and increases the electrophilicity of the carbon atoms in the epoxide ring. In an unsymmetrical epoxide, the more substituted carbon (the one bearing two methyl groups) develops a greater partial positive charge because it can better stabilize the developing positive charge, leading to an SN1-like character in the transition state.
\ce{ \begin{center} \begin{tikzpicture}[scale=0.8, xscale=1.2, yscale=1.2] \node (O) at (0,0) {O}; \node (C1) at (1,0) {C}; \node (C2) at (0.5, -0.866) {CH$_2$}; \node (CH3a) at (1.5, 0.5) {CH$_3$}; \node (CH3b) at (1.5, -0.5) {CH$_3$}; \draw (O) -- (C1); \draw (O) -- (C2); \draw (C1) -- (C2); \draw (C1) -- (CH3a); \draw (C1) -- (CH3b); \node at (2.5,0) {\large{+}}; \node at (3.5,0) {AlCl$_3$}; \node at (4.5,0) {$\rightleftharpoons$}; \node (O_act) at (5.5,0) {O$^+$}; \node (C1_act) at (6.5,0) {C}; \node (C2_act) at (6, -0.866) {CH$_2$}; \node (CH3a_act) at (7, 0.5) {CH$_3$}; \node (CH3b_act) at (7, -0.5) {CH$_3$}; \node (AlCl3_coord) at (5.5, 0.7) {AlCl$_3^-$}; \draw (O_act) -- (C1_act); \draw (O_act) -- (C2_act); \draw (C1_act) -- (C2_act); \draw (C1_act) -- (CH3a_act); \draw (C1_act) -- (CH3b_act); \draw (O_act) -- (AlCl3_coord); \end{tikzpicture} \end{center} } -
Step 2: Nucleophilic Attack by Grignard Reagent (R-MgX) The
R-group from the Grignard reagent acts as a strong nucleophile. Under Lewis acid catalysis, the nucleophile attacks the more substituted carbon of the activated epoxide. This is because the transition state has significant carbocation character at the more substituted carbon, which is more stable. The bond between the more substituted carbon and the oxygen breaks, and the oxygen remains bonded to the less substituted carbon (CH2).\ce{ \begin{center} \begin{tikzpicture}[scale=0.8, xscale=1.2, yscale=1.2] \node (O_act) at (0,0) {O$^+$}; \node (C1_act) at (1,0) {C}; \node (C2_act) at (0.5, -0.866) {CH$_2$}; \node (CH3a_act) at (1.5, 0.5) {CH$_3$}; \node (CH3b_act) at (1.5, -0.5) {CH$_3$}; \node (AlCl3_coord) at (0, 0.7) {AlCl$_3^-$}; \draw (O_act) -- (C1_act); \draw (O_act) -- (C2_act); \draw (C1_act) -- (C2_act); \draw (C1_act) -- (CH3a_act); \draw (C1_act) -- (CH3b_act); \draw (O_act) -- (AlCl3_coord); \node at (2.5,0) {\large{+}}; \node at (3.5,0) {R-MgX}; \node at (4.5,0) {$\longrightarrow$}; \node (C_prod) at (6,0) {C}; \node (R_prod) at (5.5, 0.5) {R}; \node (CH3a_prod) at (6.5, 0.5) {CH$_3$}; \node (CH3b_prod) at (6.5, -0.5) {CH$_3$}; \node (CH2_prod) at (5.5, -0.5) {CH$_2$}; \node (O_intermediate) at (5, -1) {O$^{-}$AlCl$_3$}; \draw (R_prod) -- (C_prod); \draw (CH3a_prod) -- (C_prod); \draw (CH3b_prod) -- (C_prod); \draw (C_prod) -- (CH2_prod); \draw (CH2_prod) -- (O_intermediate); \end{tikzpicture} \end{center} }The intermediate formed is an alkoxide complex.
-
Step 3: Acidic Workup (H+) The alkoxide intermediate is protonated by the acid (H+) during the workup to form the final alcohol.
\ce{ \begin{center} \begin{tikzpicture}[scale=0.8, xscale=1.2, yscale=1.2] \node (C_prod) at (0,0) {C}; \node (R_prod) at (-0.5, 0.5) {R}; \node (CH3a_prod) at (0.5, 0.5) {CH$_3$}; \node (CH3b_prod) at (0.5, -0.5) {CH$_3$}; \node (CH2_prod) at (-0.5, -0.5) {CH$_2$}; \node (O_intermediate) at (-1, -1) {O$^{-}$AlCl$_3$}; \draw (R_prod) -- (C_prod); \draw (CH3a_prod) -- (C_prod); \draw (CH3b_prod) -- (C_prod); \draw (C_prod) -- (CH2_prod); \draw (CH2_prod) -- (O_intermediate); \node at (1.5,0) {\large{+}}; \node at (2.5,0) {H$^+$}; \node at (3.5,0) {$\longrightarrow$}; \node (C_final) at (5,0) {C}; \node (R_final) at (4.5, 0.5) {R}; \node (CH3a_final) at (5.5, 0.5) {CH$_3$}; \node (CH3b_final) at (5.5, -0.5) {CH$_3$}; \node (CH2_final) at (4.5, -0.5) {CH$_2$}; \node (OH_final) at (4, -1) {OH}; \draw (R_final) -- (C_final); \draw (CH3a_final) -- (C_final); \draw (CH3b_final) -- (C_final); \draw (C_final) -- (CH2_final); \draw (CH2_final) -- (OH_final); \end{tikzpicture} \end{center} }
The final product is a primary alcohol with the general structure: R-C(CH3)2-CH2-OH.
Explanation of the Solution:
The reaction involves the Lewis acid-catalyzed ring-opening of 2,2-dimethyloxirane by a Grignard reagent. The Lewis acid (AlCl3) activates the epoxide oxygen, making the carbons more electrophilic. Due to the stability of the developing positive charge, the nucleophilic attack by the 'R' group of the Grignard reagent occurs at the more substituted carbon (the one with two methyl groups). This opens the epoxide ring, forming an alkoxide intermediate. Subsequent acidic workup protonates the alkoxide to yield a primary alcohol.
