Question
Question: CH3 A benzene ring with a methyl group (CH3) attached at the top is shown. This molecule is labeled...
CH3
A benzene ring with a methyl group (CH3) attached at the top is shown. This molecule is labeled as a reactant. It reacts with Br2 (bromine) in the presence of Fe (iron) and under dark conditions, indicated by 'Dark' written below 'Fe'. An arrow points from the reactants to 'Product', indicating the formation of a product.
- Br2 Fe Dark → Product
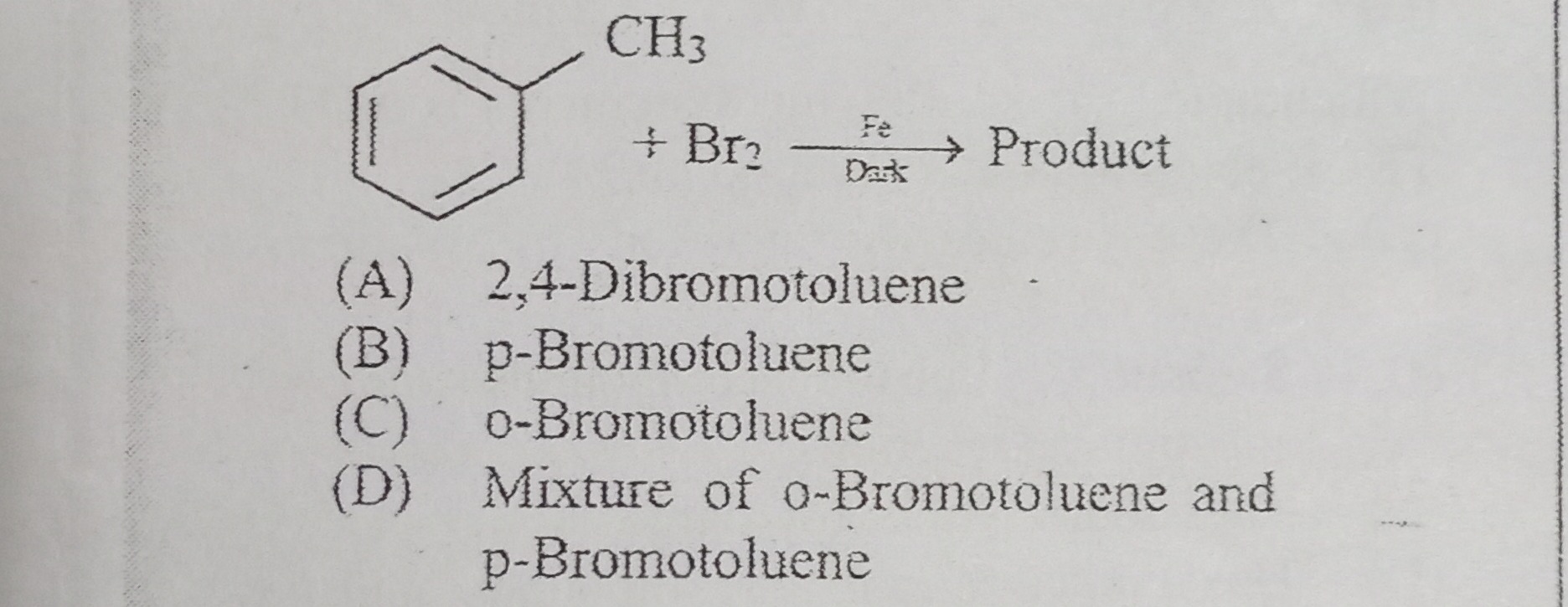
2,4-Dibromotoluene
p-Bromotoluene
o-Bromotoluene
Mixture of o-Bromotoluene and p-Bromotoluene
Mixture of o‑Bromotoluene and p‑Bromotoluene
Solution
A benzene ring with a CH₃ group undergoes electrophilic aromatic substitution. The CH₃ group is an ortho/para director, so bromination occurs at the ortho and para positions. Under controlled (dark) conditions with a Fe catalyst (which avoids radical pathways), both o‑ and p‑bromotoluene are formed, with the para product being major but still yielding a mixture.
