Question
Question: How many stereoisomers are possible for 1,3-diethylcyclopentane?...
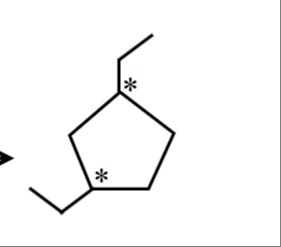
How many stereoisomers are possible for 1,3-diethylcyclopentane?
2
3
4
6
3
Solution
The molecule in question is 1,3-diethylcyclopentane. The asterisks indicate two chiral centers at positions 1 and 3 of the cyclopentane ring. For a molecule with two chiral centers, the maximum number of stereoisomers is 22=4. These are (1R, 3R), (1S, 3S), (1R, 3S), and (1S, 3R).
We need to consider the cis and trans isomers.
-
Cis-1,3-diethylcyclopentane: In the cis isomer, both ethyl groups are on the same side of the cyclopentane ring. If the substituents are identical and achiral, the cis isomer of a 1,3-disubstituted cyclopentane is a meso compound, meaning it is achiral despite having chiral centers due to the presence of an internal plane of symmetry. In this case, the configuration (1R, 3S) is identical to (1S, 3R) due to the plane of symmetry. Therefore, the cis isomer exists as a single, achiral stereoisomer (meso compound).
-
Trans-1,3-diethylcyclopentane: In the trans isomer, the ethyl groups are on opposite sides of the cyclopentane ring. This isomer does not possess a plane of symmetry.
- The configurations (1R, 3R) and (1S, 3S) are enantiomers of each other.
- The configurations (1R, 3S) and (1S, 3R) are enantiomers of each other. Furthermore, the pair of enantiomers (1R, 3R)/(1S, 3S) are diastereomers of the pair (1R, 3S)/(1S, 3R). Thus, the trans isomer exists as two pairs of enantiomers, which are diastereomeric to each other. This accounts for two distinct stereoisomers for the trans form.
Combining the cis and trans isomers:
- Cis isomer: 1 stereoisomer (meso)
- Trans isomer: 2 stereoisomers (a pair of enantiomers)
Therefore, the total number of stereoisomers for 1,3-diethylcyclopentane is 1+2=3.
