Question
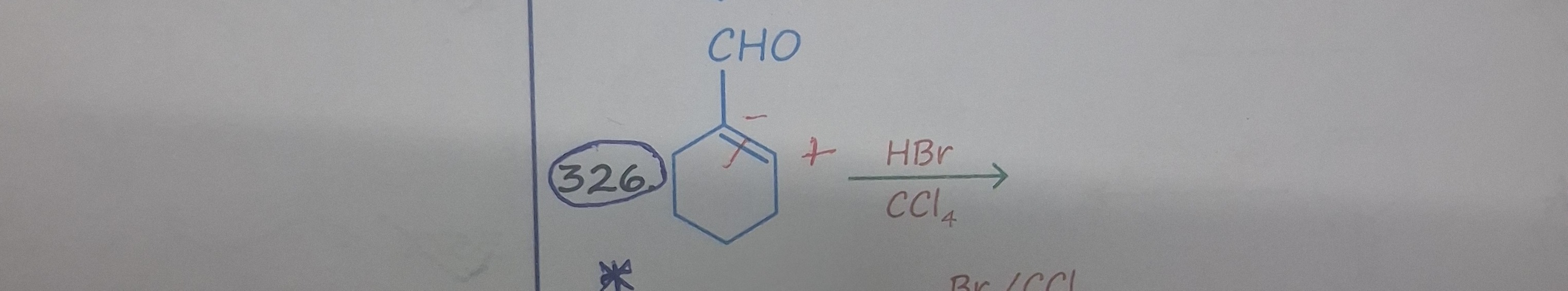
Question: The reaction involves the electrophilic addition of HBr to a cyclic alkene. The reactant is a cycloh...
The reaction involves the electrophilic addition of HBr to a cyclic alkene. The reactant is a cyclohexene derivative with a CHO group attached to one carbon of the double bond and a negative charge on the other carbon of the double bond. The negative charge on the vinylic carbon makes it highly nucleophilic. Therefore, the proton (H+) from HBr will preferentially attack this carbon. This leads to the formation of a carbocation on the adjacent vinylic carbon, which is attached to the CHO group. The bromide ion (Br-) then attacks this carbocation, resulting in the formation of the product.
1-bromo-1-formylcyclohexane
Solution
The reaction proceeds via electrophilic addition. The nucleophilic carbon with the negative charge attacks the proton from HBr, forming a carbocation on the adjacent carbon bearing the formyl group. The bromide ion then attacks this carbocation to yield the final product, 1-bromo-1-formylcyclohexane.
