Question
Question: The given compound is a substituted benzene derivative. We can identify the substituents as a methyl...
The given compound is a substituted benzene derivative. We can identify the substituents as a methyl group (−CH3), a chlorine atom (−Cl), and a nitro group (−NO2).
According to IUPAC nomenclature rules for benzene derivatives:
- If a substituent present on the benzene ring gives it a common name accepted as a parent name, that name should be used. Toluene (methylbenzene) is such a parent name.
- When toluene is the parent compound, the methyl group is assigned position 1.
- The numbering of the ring proceeds in a direction that gives the lowest possible locants to the other substituents.
- The substituents are then listed in alphabetical order, preceded by their locant numbers.
In the given structure:
- The methyl group is at position 1.
- The chlorine atom is attached to the carbon adjacent to the methyl group, which is position 2.
- The nitro group is attached to the carbon opposite to the methyl group, which is position 4.
The substituents are 'chloro' and 'nitro'. In alphabetical order, 'chloro' comes before 'nitro'. Therefore, the IUPAC name of the compound is 2-chloro-4-nitrotoluene.
SMILES Representation: Cc1cc(Cl)ccc1[N+](=O)[O−]
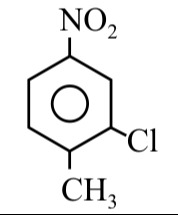
2-chloro-4-nitrotoluene
Solution
The compound is a substituted benzene. The presence of a methyl group allows us to use toluene as the parent name, with the methyl group at position 1. The chlorine atom is at position 2 and the nitro group is at position 4. Following alphabetical order for substituents, the IUPAC name is 2-chloro-4-nitrotoluene.
