Question
Question: Arrange the following compounds in order of decreasing reactivity towards nucleophilic aromatic subs...
Arrange the following compounds in order of decreasing reactivity towards nucleophilic aromatic substitution with sodium methoxide: I. Chlorobenzene II. 1-chloro-2-nitrobenzene III. 1-chloro-4-nitrobenzene IV. 1-chloro-2,4-dinitrobenzene (Compound A)
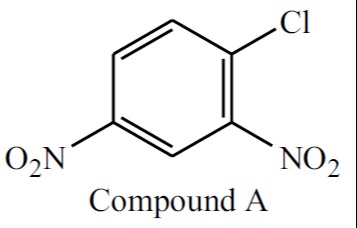
A
IV > III > II > I
B
I > II > III > IV
C
IV > II > III > I
D
III > IV > II > I
Answer
IV > III > II > I
Explanation
Solution
Nucleophilic aromatic substitution (SNAr) is facilitated by electron-withdrawing groups (EWGs) at the ortho and/or para positions relative to the leaving group (chlorine in this case). These EWGs stabilize the negatively charged intermediate, known as the Meisenheimer complex.
- Compound IV (1-chloro-2,4-dinitrobenzene): Has two strong EWGs (nitro groups) at both ortho and para positions, providing maximum activation.
- Compound III (1-chloro-4-nitrobenzene): Has one strong EWG (nitro group) at the para position, allowing significant resonance stabilization.
- Compound II (1-chloro-2-nitrobenzene): Has one strong EWG (nitro group) at the ortho position, providing activation, though generally less resonance stabilization than the para isomer.
- Compound I (Chlorobenzene): Has no EWGs, making it the least reactive.
Therefore, the order of decreasing reactivity is IV > III > II > I.
