Question
Question: The provided image depicts a chemical structure representing a carbocation. The skeletal structure c...
The provided image depicts a chemical structure representing a carbocation. The skeletal structure can be interpreted as a carbon atom bonded to a methyl group (CH3), a nitro group (NO2), and bearing a positive charge. This can be written as CH3-C(+)-NO2.
-
Identification of the species: The structure represents a carbocation, specifically a primary carbocation because the positively charged carbon atom is bonded to only one other carbon atom (in the methyl group).
-
Hybridization and Geometry: The positively charged carbon atom is bonded to three atoms (one carbon and one nitrogen) and has no lone pairs. Therefore, it is sp2 hybridized and has a trigonal planar geometry.
-
Stability: Carbocations are stabilized by electron-donating groups and destabilized by electron-withdrawing groups. The nitro group (-NO2) is a strong electron-withdrawing group due to both inductive and resonance effects. This electron-withdrawing nature significantly destabilizes the carbocation.
-
Resonance Stabilization (Special Case): While the NO2 group is generally electron-withdrawing, there can be resonance stabilization in certain cases involving carbocations. In the structure CH3-C(+)-NO2, the positively charged carbon atom has an empty p orbital. The nitro group itself has resonance structures: O=N+=O(-) <--> -O-N+=O. If the carbocation carbon is bonded to the nitrogen atom, the electron density from the nitro group can be delocalized. Specifically, the lone pair on the negatively charged oxygen atom in one resonance form of the nitro group can be donated to the empty p orbital of the carbocation carbon. This leads to a resonance structure: CH3−C+−N(=O)O−⟷CH3−C−N(=O)=O ∣ O− In this resonance structure, the positive charge is delocalized onto the oxygen atom. This resonance stabilization can contribute to the stability of the carbocation.
-
Overall Stability: The overall stability of this carbocation is a result of the destabilizing inductive effect of the NO2 group and the resonance stabilization it provides. Compared to a simple primary carbocation (like CH3-CH2-CH2+), the presence of the NO2 group directly attached to the carbocation center makes it significantly less stable due to the strong electron-withdrawing nature. However, the resonance stabilization cannot be ignored.
Since no specific question was asked, we identify the species as a carbocation and discuss its structure and stability.
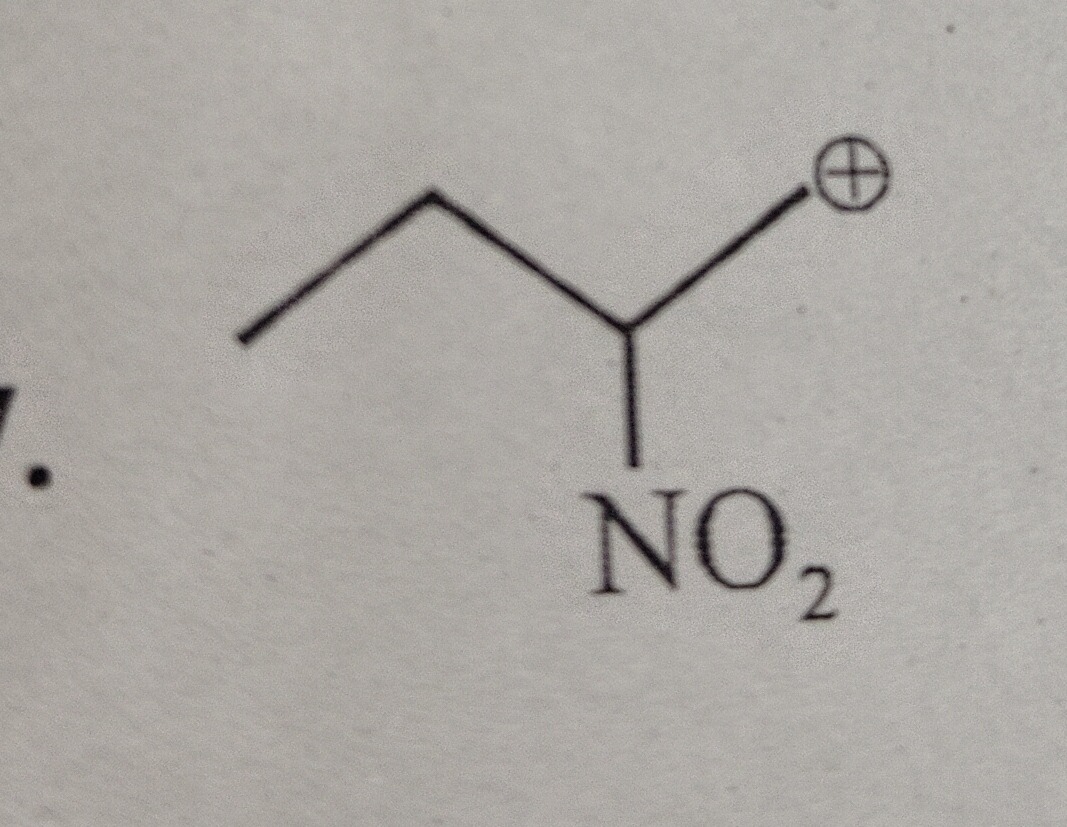
The species is a carbocation: CH3-C(+)-NO2.
Solution
The structure shows a carbon atom with a positive charge, bonded to a methyl group and a nitro group, thus identifying it as a carbocation, specifically CH3-C(+)-NO2. The nitro group is electron-withdrawing, which generally destabilizes carbocations. However, resonance stabilization involving the nitro group can delocalize the positive charge onto an oxygen atom, contributing to its stability.
