Question
Question: ...
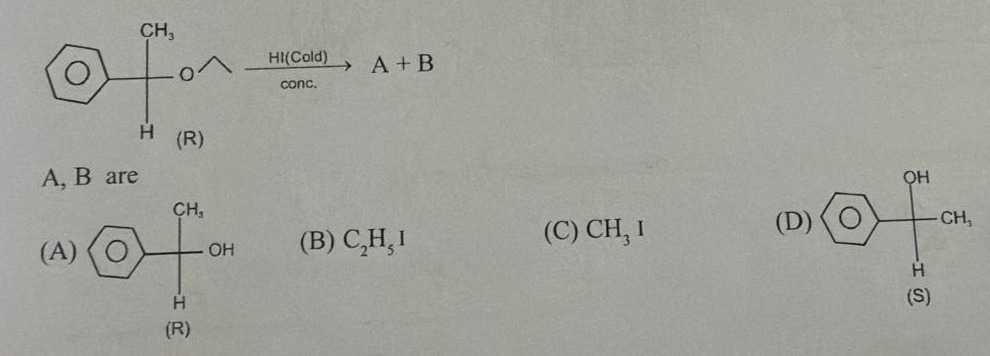
A
(R)-1-phenylethanol
B
C2H5I (iodoethane)
C
CH3I (iodomethane)
D
(S)-1-phenylethanol
Answer
A and B are (A) and (B)
Explanation
Solution
The reaction is the cleavage of 1-ethoxy-1-phenylethane with concentrated HI. The ether is unsymmetrical. The chiral carbon is secondary and benzylic. The ethyl group is primary. The reaction proceeds via SN2 mechanism where the iodide ion attacks the less sterically hindered primary carbon (ethyl group). This breaks the C-O bond of the ethyl group, forming iodoethane and 1-phenylethanol. The chiral center is not involved in the bond breaking or formation, so its configuration is retained. Since the starting ether is (R), the 1-phenylethanol formed is also (R). Therefore, the products are (R)-1-phenylethanol and iodoethane.
