Question
Question: Identify the functional group and parent chain. Number the parent chain. Determine the IUPAC name. D...
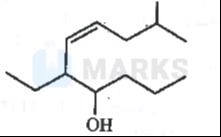
Identify the functional group and parent chain. Number the parent chain. Determine the IUPAC name. Determine stereochemistry of the double bond.
(E)-5-ethyl-2-methylhept-3-en-3-ol
(Z)-5-ethyl-2-methylhept-3-en-3-ol
5-ethyl-2-methylhept-3-en-3-ol
2-ethyl-5-methylhept-3-en-3-ol
(E)-5-ethyl-2-methylhept-3-en-3-ol
Solution
The molecule contains a hydroxyl (-OH) group and a carbon-carbon double bond. The longest continuous carbon chain including the hydroxyl group is a 7-carbon chain (heptene). Numbering starts from the end that gives the lowest locant to the hydroxyl group, which is carbon 3. The double bond is between carbons 3 and 4. There is a methyl substituent on carbon 2 and an ethyl substituent on carbon 5. The parent chain is therefore hept-3-ene. The hydroxyl group is on carbon 3, making it hept-3-en-3-ol. The substituents give the name 5-ethyl-2-methylhept-3-en-3-ol.
To determine the stereochemistry of the double bond (between C3 and C4): On C3, the groups are -OH and -CH(CH3)2. Priority: -OH > -CH(CH3)2. On C4, the groups are -CH3 and -CH(CH2CH3). Priority: -CH(CH2CH3) > -CH3. In the given structure, the higher priority groups (-OH on C3 and -CH(CH2CH3) on C4) are on opposite sides of the double bond. Therefore, the configuration is E (entgegen).
The full IUPAC name is (E)-5-ethyl-2-methylhept-3-en-3-ol.
