Question
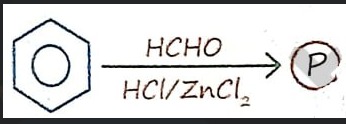
Question: The reaction shown is the Blanc chloromethylation of benzene. Benzene reacts with formaldehyde (HCHO...
The reaction shown is the Blanc chloromethylation of benzene. Benzene reacts with formaldehyde (HCHO) and hydrogen chloride (HCl) in the presence of zinc chloride (ZnCl2), a Lewis acid catalyst, to undergo electrophilic aromatic substitution. This process introduces a chloromethyl group (-CH2Cl) onto the benzene ring, forming benzyl chloride.
Benzene+CH2O+HClZnCl2Benzyl chloride
Answer
Benzyl chloride
Explanation
Solution
The Blanc chloromethylation is an electrophilic aromatic substitution reaction where benzene reacts with formaldehyde and hydrogen chloride in the presence of a Lewis acid catalyst like zinc chloride to form benzyl chloride.
