Question
Question: The provided image depicts a chemical species which is a carbocation. It consists of two phenyl ring...
The provided image depicts a chemical species which is a carbocation. It consists of two phenyl rings connected by a single bond. The positive charge is located on a carbon atom of one of the phenyl rings, specifically at the position where it is attached to the other phenyl ring (ipso position). This carbocation is a phenyl cation substituted at the ipso position with another phenyl group.
The stability of this carbocation is influenced by resonance. The positive charge can be delocalized through the pi system of the first phenyl ring to the ortho and para positions. The phenyl substituent attached at the ipso position can donate electron density through resonance, further stabilizing the carbocation. While phenyl cations are generally unstable, this resonance stabilization significantly enhances its stability. The first phenyl ring, with 4 pi electrons and an empty p-orbital on the positively charged carbon, is not aromatic.
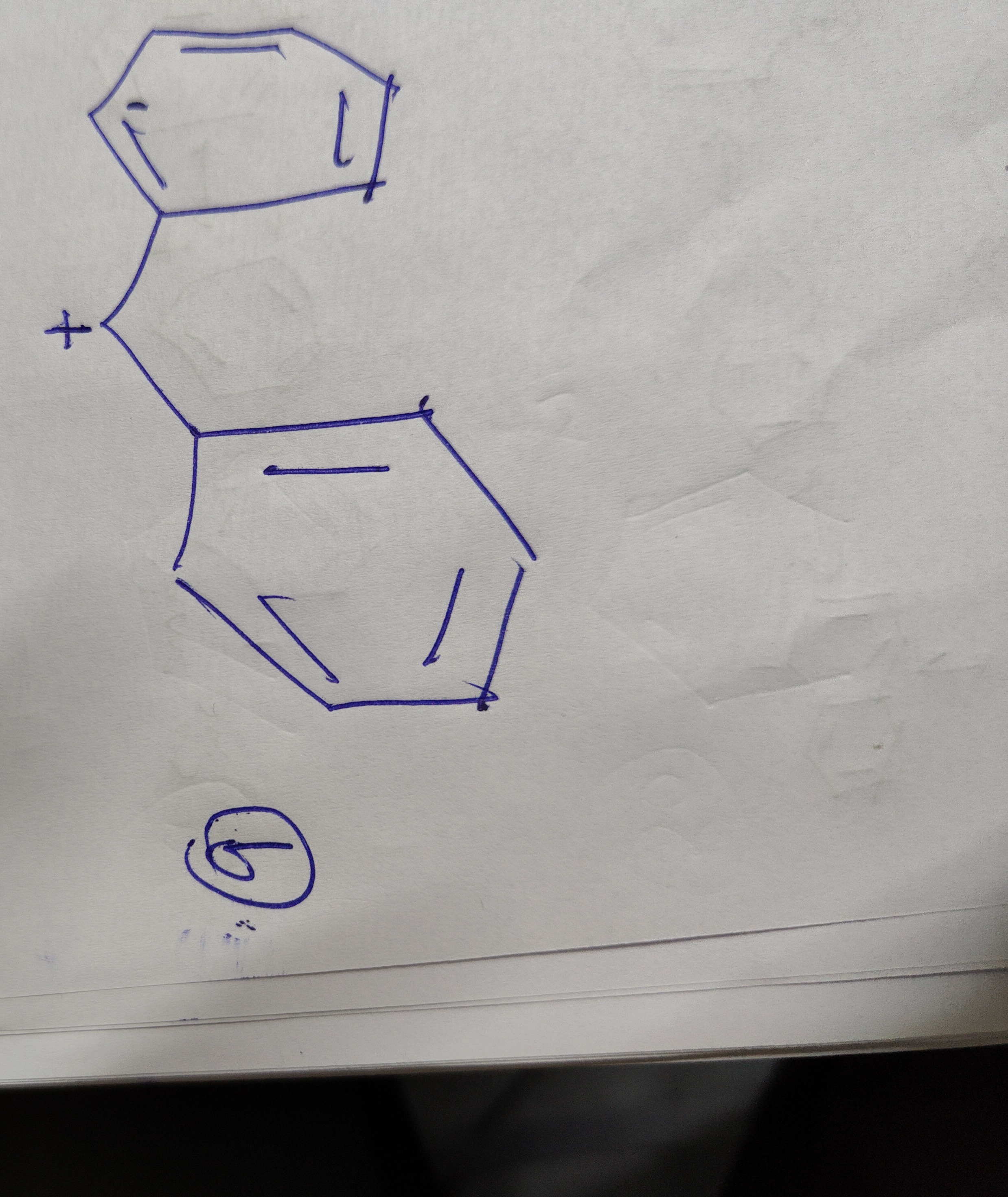
The carbocation is resonance-stabilized.
Solution
The carbocation is stabilized by the delocalization of the positive charge through the pi system of the adjacent phenyl ring. The phenyl substituent at the ipso position also contributes to this stabilization via resonance. Although phenyl cations are generally unstable, the extensive resonance delocalization in this specific structure significantly enhances its stability.
