Question
Question: What is the major product formed in the following reaction?...
What is the major product formed in the following reaction?
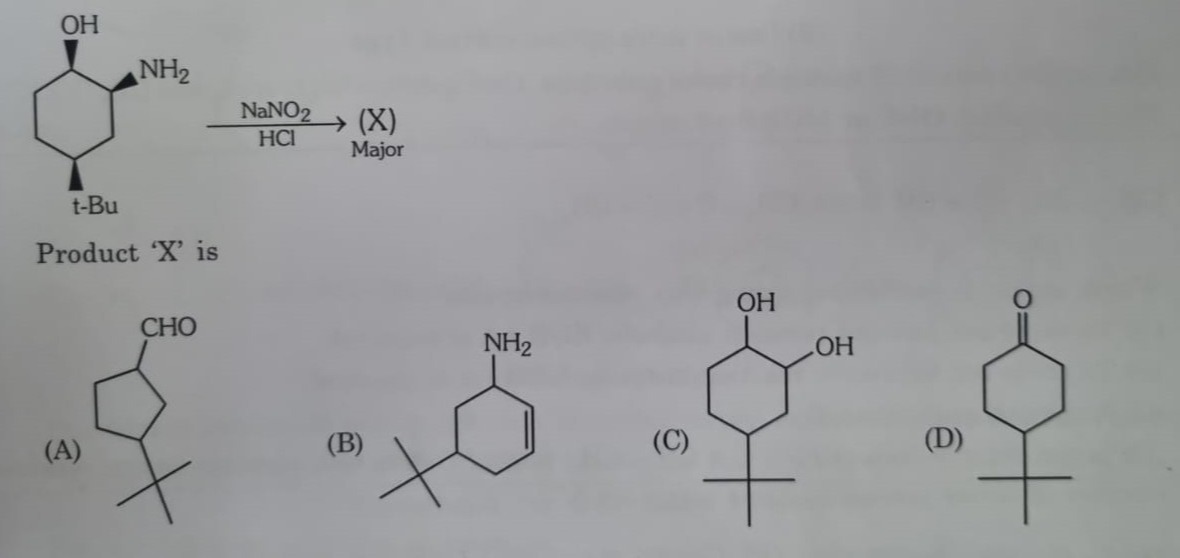
Cyclopentanecarbaldehyde derivative with a tert-butyl group
Cyclohexene derivative with a tert-butyl group
Cyclohexanone derivative with a tert-butyl group
Bicyclo[3.1.0]hexane derivative with a tert-butyl group
Cyclopentanecarbaldehyde derivative with a tert-butyl group
Solution
The reaction involves treating a primary amine with NaNO2 and HCl, generating nitrous acid (HNO2). Nitrous acid reacts with primary aliphatic amines to form unstable diazonium salts, which decompose to form a carbocation, nitrogen gas, and water. The carbocation intermediate can undergo substitution, elimination, or rearrangement.
The starting material is (1S,2R,4S)-2-amino-4-(tert-butyl)cyclohexan-1-ol. The amino group is on C2, the hydroxyl group is on C1, and the tert-butyl group is on C4.
The primary amine at C2 converts to a diazonium ion, forming a carbocation at C2 upon decomposition. The carbocation at C2 is adjacent to C1 (with a hydroxyl group) and C3. The hydroxyl group on C1 can lead to ring contraction via a 1,2-migration of the C1-C6 bond to the carbocation at C2. This forms a five-membered ring with a positive charge on C1.
The migration of the C1-C6 bond to C2 forms a five-membered ring, and the positive charge shifts to C1. The resulting five-membered ring contains carbons C1, C2, C3, C4, C5, and C6, where C2 is now bonded to C6. Carbon C1 has the OH group and the positive charge. Carbon C4 has the t-Bu group. The positive charge on C1 is adjacent to the oxygen of the hydroxyl group and can lose a proton to form a carbonyl group (aldehyde).
Therefore, the major product is a cyclopentanecarbaldehyde derivative with a tert-butyl group.
