Question
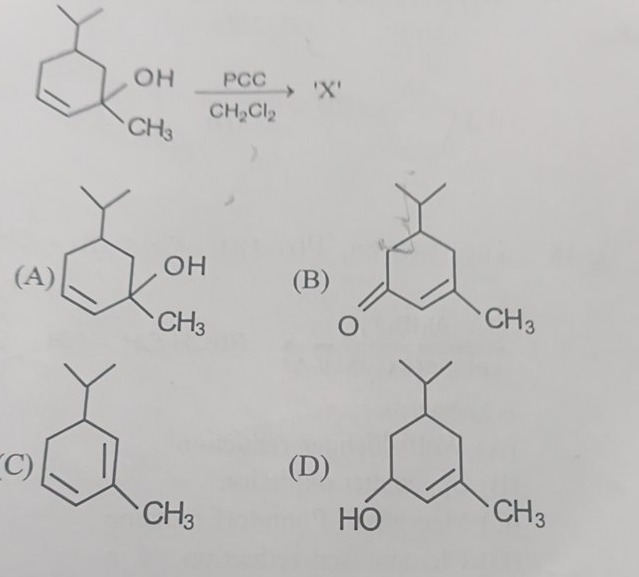
Question: The starting material is a tertiary allylic alcohol. Pyridinium Chlorochromate (PCC) in dichlorometh...
The starting material is a tertiary allylic alcohol. Pyridinium Chlorochromate (PCC) in dichloromethane (CH2Cl2) is a mild oxidizing agent that oxidizes primary alcohols to aldehydes and secondary alcohols to ketones. Tertiary alcohols are generally resistant to oxidation under these conditions. However, tertiary allylic alcohols can undergo oxidation, typically leading to the formation of α,β-unsaturated ketones.
In the given starting material, the hydroxyl group (-OH) and a methyl group (-CH3) are attached to the same tertiary carbon atom, which is also allylic to the double bond in the cyclohexene ring. Upon oxidation with PCC, the tertiary allylic alcohol is converted into a ketone. The carbon atom bearing the -OH group becomes a carbonyl carbon (C=O), and the methyl group remains attached to it. The double bond, which was allylic to the alcohol, becomes conjugated with the newly formed carbonyl group.
Let's analyze the structure of the starting material and option (B). The starting material is 1-hydroxy-1-methyl-4-isopropylcyclohex-2-ene. The reaction with PCC oxidizes the tertiary allylic alcohol at C1 to a ketone. Thus, C1 becomes a carbonyl group (C=O). The methyl group remains attached to C1. The double bond is between C2 and C3. The isopropyl group is at C4. The product formed is 4-isopropyl-2-methylcyclohex-2-en-1-one.
4-isopropyl-1-methylcyclohex-2-en-1-one
4-isopropyl-2-methylcyclohex-2-en-1-one
4-isopropyl-1-methylcyclohex-3-en-1-one
4-isopropylcyclohex-2-en-1-one
4-isopropyl-2-methylcyclohex-2-en-1-one
Solution
The reaction involves the oxidation of a tertiary allylic alcohol using Pyridinium Chlorochromate (PCC). Tertiary allylic alcohols, under these conditions, typically oxidize to α,β-unsaturated ketones. The starting material is 1-hydroxy-1-methyl-4-isopropylcyclohex-2-ene. The tertiary carbon bearing the hydroxyl group and the methyl group is allylic to the double bond. Oxidation converts the hydroxyl group into a carbonyl group (ketone). The methyl group remains attached to the carbonyl carbon. The double bond shifts to become conjugated with the carbonyl group. Therefore, the product is 4-isopropyl-2-methylcyclohex-2-en-1-one.
