Question
Question: Which of the following is the major product of the acid-catalyzed hydration of 3-methylcyclopropenon...
Which of the following is the major product of the acid-catalyzed hydration of 3-methylcyclopropenone?
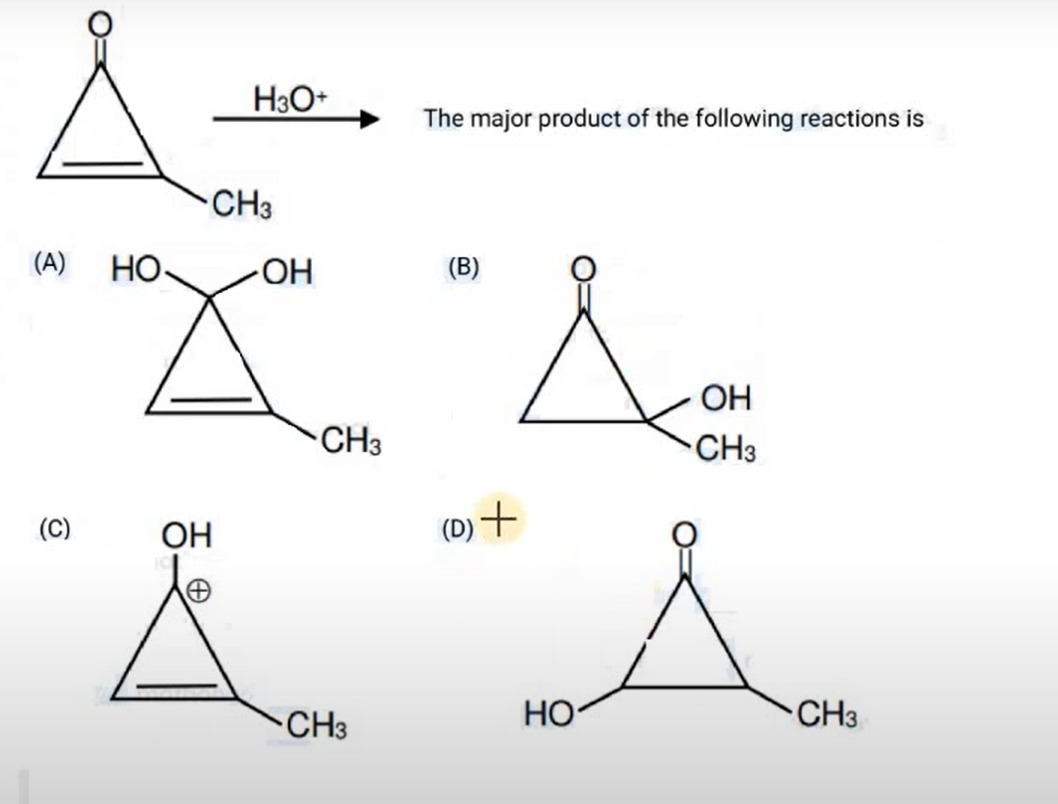
A geminal diol formed by the addition of two hydroxyl groups to the carbonyl carbon, with the double bond and methyl group intact.
An enol formed by the addition of a hydroxyl group to one of the carbons of the double bond.
A carbocation intermediate.
A cyclopropane ring with a carbonyl, a hydroxyl, and a methyl group, implying reduction of the double bond.
A geminal diol formed by the addition of two hydroxyl groups to the carbonyl carbon, with the double bond and methyl group intact.
Solution
The acid-catalyzed hydration of a ketone, such as 3-methylcyclopropenone, results in the formation of a geminal diol. The carbonyl oxygen is first protonated, increasing the electrophilicity of the carbonyl carbon. Water then attacks the carbonyl carbon, leading to the addition of two hydroxyl groups to the same carbon. Cyclopropenones are highly strained and electron-deficient, making them susceptible to nucleophilic attack at the carbonyl carbon. The double bond and the methyl substituent remain unchanged under these reaction conditions. Therefore, the major product is the geminal diol formed by hydration of the C=O bond.
