Question
Question: What reagents are required for the following transformation?...
What reagents are required for the following transformation?
- Fe/HCl; 2. NaNO2/HCl; 3. CuCN; 4. H+/H2O; 5. Br2/CH3COOH
- KMnO4; 2. Fe/HCl; 3. NaNO2/HCl; 4. CuBr
- Fe/HCl; 2. NaNO2/HCl; 3. CuBr; 4. KMnO4
- KMnO4; 2. Fe/HCl; 3. NaNO2/HCl; 4. CuCN; 5. H+/H2O
- Fe/HCl; 2. NaNO2/HCl; 3. CuCN; 4. H+/H2O; 5. Br2/CH3COOH
Solution
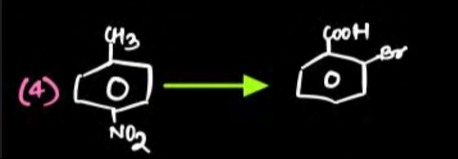
The transformation of 1-methyl-2-nitrobenzene to 2-bromo-3-methylbenzoic acid involves several key steps:
-
Reduction of the nitro group to an amino group: The nitro group (−NO2) is reduced to an amino group (−NH2) using a reducing agent like iron in acidic medium (Fe/HCl). This converts 1-methyl-2-nitrobenzene to 2-methylaniline.
-
Diazotization of the amino group: The amino group is converted into a diazonium salt (−N2+) by reaction with sodium nitrite (NaNO2) in the presence of a strong acid (e.g., HCl) at low temperatures (0−5∘C). This yields 2-methylbenzenediazonium chloride.
-
Sandmeyer reaction to introduce a nitrile group: The diazonium salt is then reacted with copper(I) cyanide (CuCN) to replace the diazonium group with a nitrile group (−CN). This step yields 2-methylbenzonitrile.
-
Hydrolysis of the nitrile group to a carboxylic acid group: The nitrile group is hydrolyzed under acidic or basic conditions to form a carboxylic acid group (−COOH). Hydrolysis with acid (H+/H2O) converts 2-methylbenzonitrile to 2-methylbenzoic acid.
-
Bromination: The final step is to introduce a bromine atom at the correct position. In 2-methylbenzoic acid, the methyl group is at position 2 and the carboxylic acid group is at position 1. We need to introduce bromine at position 3 (ortho to the methyl group and meta to the carboxylic acid group). Bromination of 2-methylbenzoic acid with Br2 in acetic acid (CH3COOH) can lead to the desired substitution at position 3, although regioselectivity can be complex due to competing directing effects. The methyl group is activating and ortho/para directing, while the carboxylic acid group is deactivating and meta directing. The position ortho to the methyl group (position 3) is also ortho to the deactivating carboxylic acid group, but under appropriate conditions, this substitution can be achieved.
Therefore, the sequence of reagents is: 1. Fe/HCl; 2. NaNO2/HCl; 3. CuCN; 4. H+/H2O; 5. Br2/CH3COOH.
